Multiple pathways control protein kinase C phosphorylation - PubMed (original) (raw)
Review
Multiple pathways control protein kinase C phosphorylation
D B Parekh et al. EMBO J. 2000.
No abstract available
Figures
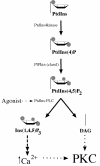
Fig. 1. The classical pathway of PKC activation. The scheme illustrates the production of the immediate precursor lipid PtdIns(4,5)P2 from its parent lipid PtdIns. Various agonists are linked to the phospholipases (PtdIns-PLC) that can cleave PtdIns(4,5)P2 to diacylglycerol (DAG) and the calcium mobilizer Ins(1,4,5)P3. Calcium can affect the cPKC class by promoting membrane recruitment, but the key allosteric activator at the membrane for both cPKC and nPKC isotypes is DAG.
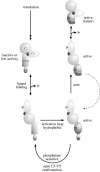
Fig. 2. Model for the phosphorylation of PKC. PKC is represented by a regulatory domain, comprising a C1 domain, C2 domain and pseudosubstrate site (black circle) and a catalytic domain (C3/4) with a C-terminal, V5, extension. The unphosphorylated primary translation product is predicted to have little or no activity. On ligand binding at the membrane, PKC becomes a substrate for kinases acting upon activation loop sites (for PKCα the T497 site) and hydrophobic C–terminal sites (for PKCα the S657 site). Following subsequent autophosphorylation (for PKCα the T638 site), the kinase domain is in a closed conformation that confers stability and phosphatase resistance. Ligand dissociation allows the kinase to diffuse away from the membrane but to remain in a phosphorylated state. The latent kinase can then be recruited back to the membrane and reactivated by DAG alone.
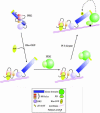
Fig. 3. AGC kinase domain alignment. The kinase domains of PKCα/δ/ζ, the PKC-related kinase PRK1, PKBα and p70S6kinase α (p70α) are aligned from their first conserved kinase sub-domain (I) through sub-domain X, to the region covering the C-terminal extension. The kinase sub-domains are indicated by the shaded areas, with regions I–X displayed with an increasingly darker hue. The three phosphorylation sites (or acidic residues) discussed in the text are indicated by the red bars. The activation loop is indicated by the dark purple block, and the hydrophobic C-terminal phosphorylation site is also boxed in dark purple.
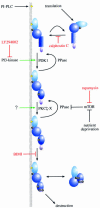
Fig. 4. Rho-dependent PRK activation via PDK1. In the unliganded state, PRK is shown to be in an inactive conformation, with the pseudosubstrate site(s) in the HR1 domain interacting with the catalytic domain. On Rho binding to the HR1 motif in a membrane compartment, the kinase domain is released and can express catalytic activity, albeit at a low level. The exposed kinase domain can interact with PDK1 through its PIF motif (see text) and, on its association with PtdIns(3,4,5)P3, PDK1 will phosphorylate and activate PRK. The domains illustrated are summarized in the figure.
Fig. 5. A general scheme of PKC controls. The figure illustrates the upstream inputs to membrane recruitment and phosphorylation of PKC that are required to generate the ‘mature’ phosphorylated form. For reasons of clarity, this is a simplified view of the process that excludes the action of PKC-interacting proteins that are likely to play roles in localization and membrane targeting. Some of the inhibitors that can block specific steps in the input pathways are included (in red), as well as the kinases and phosphatases (undefined) predicted to play key roles in effecting the modifications. External influences are known to control many of these steps, including activation of PtdIns-PLC, PtdIns 3-kinase and mTOR. Inputs to the aPKC complex (?) are as yet undefined.
Similar articles
- Genetic manipulation of protein kinase C in vivo.
Toker A. Toker A. Methods Mol Biol. 2003;233:475-89. doi: 10.1385/1-59259-397-6:475. Methods Mol Biol. 2003. PMID: 12840530 No abstract available. - Isozyme-specific inhibitors and activators of protein kinase C.
Schechtman D, Mochly-Rosen D. Schechtman D, et al. Methods Enzymol. 2002;345:470-89. doi: 10.1016/s0076-6879(02)45039-2. Methods Enzymol. 2002. PMID: 11665630 - Protein kinase C and its substrates.
Liu JP. Liu JP. Mol Cell Endocrinol. 1996 Jan 15;116(1):1-29. doi: 10.1016/0303-7207(95)03706-3. Mol Cell Endocrinol. 1996. PMID: 8822261 Review. No abstract available. - Dynamics and Membrane Interactions of Protein Kinase C.
Igumenova TI. Igumenova TI. Biochemistry. 2015 Aug 18;54(32):4953-68. doi: 10.1021/acs.biochem.5b00565. Epub 2015 Aug 5. Biochemistry. 2015. PMID: 26214365 Free PMC article. Review. - Protein kinase C isotypes and their specific functions: prologue.
Ohno S, Nishizuka Y. Ohno S, et al. J Biochem. 2002 Oct;132(4):509-11. doi: 10.1093/oxfordjournals.jbchem.a003249. J Biochem. 2002. PMID: 12359062 Review. No abstract available.
Cited by
- PKCη/Rdx-driven phosphorylation of PDK1: a novel mechanism promoting cancer cell survival and permissiveness for parvovirus-induced lysis.
Bär S, Rommelaere J, Nüesch JP. Bär S, et al. PLoS Pathog. 2015 Mar 5;11(3):e1004703. doi: 10.1371/journal.ppat.1004703. eCollection 2015 Mar. PLoS Pathog. 2015. PMID: 25742010 Free PMC article. - Protein kinase C delta contributes to increase in EP3 agonist-induced contraction in mesenteric arteries from type 2 diabetic Goto-Kakizaki rats.
Ishida K, Matsumoto T, Taguchi K, Kamata K, Kobayashi T. Ishida K, et al. Pflugers Arch. 2012 Apr;463(4):593-602. doi: 10.1007/s00424-012-1088-9. Epub 2012 Feb 28. Pflugers Arch. 2012. PMID: 22371141 - Novel regulation of protein kinase C-η.
Pal D, Outram SP, Basu A. Pal D, et al. Biochem Biophys Res Commun. 2012 Sep 7;425(4):836-41. doi: 10.1016/j.bbrc.2012.07.163. Epub 2012 Aug 7. Biochem Biophys Res Commun. 2012. PMID: 22892130 Free PMC article. - Adi3 is a Pdk1-interacting AGC kinase that negatively regulates plant cell death.
Devarenne TP, Ekengren SK, Pedley KF, Martin GB. Devarenne TP, et al. EMBO J. 2006 Jan 11;25(1):255-65. doi: 10.1038/sj.emboj.7600910. Epub 2005 Dec 15. EMBO J. 2006. PMID: 16362044 Free PMC article. - PKCα Modulates Epithelial-to-Mesenchymal Transition and Invasiveness of Breast Cancer Cells Through ZEB1.
Llorens MC, Rossi FA, García IA, Cooke M, Abba MC, Lopez-Haber C, Barrio-Real L, Vaglienti MV, Rossi M, Bocco JL, Kazanietz MG, Soria G. Llorens MC, et al. Front Oncol. 2019 Nov 27;9:1323. doi: 10.3389/fonc.2019.01323. eCollection 2019. Front Oncol. 2019. PMID: 31828042 Free PMC article.
References
- Alessi D.R., James, S.R., Downes, C.P., Holmes, A.B., Gaffney, P.R.J., Reese, C.B. and Cohen, P. (1997) Characterization of a 3-phosphoinositide-dependent protein-kinase which phosphorylates and activates protein-kinase B-α. Curr. Biol., 7, 261–269. - PubMed
- Alessi D.R., Kozlowski, M.T., Weng, Q.P., Morrice, N. and Avruch, J. (1998) 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro.Curr. Biol., 8, 69–81. - PubMed
- Balendran A., Casamayor, A., Deak, M., Paterson, A., Gaffney, P., Currie, R., Downes, C.P. and Alessi, D.R. (1999) PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr. Biol., 9, 393–404. - PubMed
- Bornancin F. and Parker, P.J. (1996) Phosphorylation of threonine-638 critically controls the dephosphorylation and inactivation of protein-kinase C-α. Curr. Biol., 6, 1114–1123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources