Polycystin 1 is required for the structural integrity of blood vessels - PubMed (original) (raw)
Polycystin 1 is required for the structural integrity of blood vessels
K Kim et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Autosomal dominant polycystic kidney disease (ADPKD), often caused by mutations in the PKD1 gene, is associated with life-threatening vascular abnormalities that are commonly attributed to the frequent occurrence of hypertension. A previously reported targeted mutation of the mouse homologue of PKD1 was not associated with vascular fragility, leading to the suggestion that the vascular lesion may be of a secondary nature. Here we demonstrate a primary role of PKD1 mutations in vascular fragility. Mouse embryos homozygous for the mutant allele (Pkd1(L)) exhibit s.c. edema, vascular leaks, and rupture of blood vessels, culminating in embryonic lethality at embryonic day 15.5. Kidney and pancreatic ductal cysts are present. The Pkd1-encoded protein, mouse polycystin 1, was detected in normal endothelium and the surrounding vascular smooth muscle cells. These data reveal a requisite role for polycystin 1 in maintaining the structural integrity of the vasculature as well as epithelium and suggest that the nature of the PKD1 mutation contributes to the phenotypic variance in ADPKD.
Figures
Figure 1
Targeted disruption of mouse Pkd1. (A) The genomic segment of WT and recombinant murine Pkd1. Exons (black boxes) are numbered. The position of the recombinations, the expected sizes of WT and mutant (Mut.) fragments, and the position of the external 5′ and 3′ probes are indicated. (B) Southern blots of genomic DNA extracted from ES clones, _Kpn_I-restricted, and probed with the 5′ external probe. A recombinant mutant fragment (8.1 kb) replaces the normal allele (6.8 kb) in several clones. DNA from clone #282 and #130 is shown in lanes 2 and 6. (C and D) Germ-line transmission of the mutant allele. Yolk sac DNA obtained from the offspring of F1 intercrosses was extracted, digested with _Kpn_I or _Xho_I, and probed, respectively, with the 5′ (C) or 3′ (TSC2 exon 33, 430 bp, D) probes. Fragment size is indicated by arrows. Southern blots of DNA from clone #282 (lanes 1–4 in C and 1–3 in D) and #130 (lanes 5–7, C) F2 embryos is shown. +/+, +/−, and −/− indicate WT, Pkd1+/L, and _Pkd1_L/L, respectively. (_E-_-G) Northern blot analysis. The mutant allele (15.4 kb) is found in _Pkd1_L/L (lane 3) and Pkd1+/L (lane 2) but not WT animals, who express only the WT mRNA of 14.1 kb (lane 1). The mutant but not the WT allele hybridized with the neo probe (F). (G) The normal-sized mRNA for mouse TSC2 (5.6 kb, *) in _Pkd1_L/L embryos. (H) Primary structure of polycystin 1 and the site of the predicted truncation (arrow). Mutant mouse polycystin 1 is expected to terminate after L3946 (equivalent to L3955 in human).
Figure 2
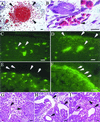
Gross phenotype of _Pkd_L/L embryos at different stages of development. Whole mounts of _Pkd_L/L (B, D, F, H, J, and K) and age-matched WT embryos (A, C, E, G, and I) are shown. Localized hemorrhages and s.c. edema (arrows) distributed over the body surface, with punctate and localized hemorrhages seen in the head (B, D, and J) and toes (H and K) of _Pkd1_L/L (J, H, and K) but not WT (A, C, E, G, and I) unstained embryos. At E15.5, fatal hemorrhages and edema are seen in _Pkd_L/L embryos (H). Staged embryos were dissected in PBS and photographed by using a Leica MZ12 microscope.
Figure 3
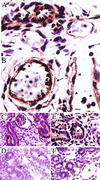
Histological analysis of tissues from _Pkd1_L/L embryos. (A) Hematoxylin and eosin stain showing red blood cells (arrowheads) outside a blood vessel in the neck region at E13.5. (B) A skin capillary at E13.5 from a region not showing gross hemorrhage. A red blood cell _(_arrowhead and Inset) is seen traversing the endothelial cell lining between two adjacent cells. (C–F) Tissue sections revealing the leak of intravascularly injected fluorescent dextran into the extravascular interstitium (D, arrows) at distant sites in a E12.5 _Pkd_L/L embryo (D and F). In the age-matched WT (C and E), dextran is retained within the vasculature (arrows). No fluorescence is seen on the basolateral side of the lining endothelium in WT embryos (E, arrows). In contrast, fluorescent dextran is seen basolaterally (F, arrows) and at intercellular junctions in the mutant. (G–I) Hematoxylin and eosin stain of a section of a normal developing kidney at E14.5 (G), showing two normal glomeruli (arrowheads). An age-matched Pkd_L/L kidney (H) shows two large glomerular cysts (arrows), and a tubular cyst (arrowhead). (I) A section of a pancreas from this Pkd_L/L embryo shows two tubular cysts (arrows) adjacent to normal-sized ducts (arrowhead). Sections shown in A, B, G, H, and I are 5 μm thick, and those in C_–_F are 4 μm thick. (Scale bars = 10 μm, except in E and F where the scale bar = 5 μm.)
Figure 4
Expression of polycystin 1 in WT and _Pkd1_L/L E14.5 mouse embryos. Polycystin 1 is detected in endothelial cells (arrowheads in A and B) as well as in vascular smooth muscle cells (arrows in A and B) by using the anti-LRR antibody. Polycystin 1 expression (horseradish peroxidase reaction product) is similar in small vessels in the region of the hind limb in WT (A) and _Pkd1_L/L mutant (B) embryos. In fetal kidneys, polycystin 1 expression is observed mainly on the apical (arrows in C and E) surfaces of WT and Pkd1_L/L renal tubules. No staining of renal tubules (arrows) from either WT or Pkd1_L/L embryos is observed in the presence of a 20-fold excess of the LRR fusion protein (D and F, respectively). * in F points to a tubular cyst. (Scale bars = 10 μm.)
Similar articles
- Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation.
Lu W, Peissel B, Babakhanlou H, Pavlova A, Geng L, Fan X, Larson C, Brent G, Zhou J. Lu W, et al. Nat Genet. 1997 Oct;17(2):179-81. doi: 10.1038/ng1097-179. Nat Genet. 1997. PMID: 9326937 - A human PKD1 transgene generates functional polycystin-1 in mice and is associated with a cystic phenotype.
Pritchard L, Sloane-Stanley JA, Sharpe JA, Aspinwall R, Lu W, Buckle V, Strmecki L, Walker D, Ward CJ, Alpers CE, Zhou J, Wood WG, Harris PC. Pritchard L, et al. Hum Mol Genet. 2000 Nov 1;9(18):2617-27. doi: 10.1093/hmg/9.18.2617. Hum Mol Genet. 2000. PMID: 11063721 - Comparison of Pkd1-targeted mutants reveals that loss of polycystin-1 causes cystogenesis and bone defects.
Lu W, Shen X, Pavlova A, Lakkis M, Ward CJ, Pritchard L, Harris PC, Genest DR, Perez-Atayde AR, Zhou J. Lu W, et al. Hum Mol Genet. 2001 Oct 1;10(21):2385-96. doi: 10.1093/hmg/10.21.2385. Hum Mol Genet. 2001. PMID: 11689485 - Autosomal dominant polycystic kidney disease (ADPKD, MIM 173900, PKD1 and PKD2 genes, protein products known as polycystin-1 and polycystin-2).
Boucher C, Sandford R. Boucher C, et al. Eur J Hum Genet. 2004 May;12(5):347-54. doi: 10.1038/sj.ejhg.5201162. Eur J Hum Genet. 2004. PMID: 14872199 Review. - Molecular genetics and mechanism of autosomal dominant polycystic kidney disease.
Wu G, Somlo S. Wu G, et al. Mol Genet Metab. 2000 Jan;69(1):1-15. doi: 10.1006/mgme.1999.2943. Mol Genet Metab. 2000. PMID: 10655152 Review.
Cited by
- Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease.
Leuenroth SJ, Okuhara D, Shotwell JD, Markowitz GS, Yu Z, Somlo S, Crews CM. Leuenroth SJ, et al. Proc Natl Acad Sci U S A. 2007 Mar 13;104(11):4389-94. doi: 10.1073/pnas.0700499104. Epub 2007 Mar 6. Proc Natl Acad Sci U S A. 2007. PMID: 17360534 Free PMC article. - Primary cilia of human endothelial cells disassemble under laminar shear stress.
Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Iomini C, et al. J Cell Biol. 2004 Mar 15;164(6):811-7. doi: 10.1083/jcb.200312133. J Cell Biol. 2004. PMID: 15024030 Free PMC article. - The polycystin-1 C-terminal fragment triggers branching morphogenesis and migration of tubular kidney epithelial cells.
Nickel C, Benzing T, Sellin L, Gerke P, Karihaloo A, Liu ZX, Cantley LG, Walz G. Nickel C, et al. J Clin Invest. 2002 Feb;109(4):481-9. doi: 10.1172/JCI12867. J Clin Invest. 2002. PMID: 11854320 Free PMC article. - Identification of a new target molecule for a cascade therapy of polycystic kidney.
Yoshida N, Yano Y, Yoshiki A, Ueno M, Deguchi N, Hirotsune S. Yoshida N, et al. Hum Cell. 2003 Jun;16(2):65-72. doi: 10.1111/j.1749-0774.2003.tb00132.x. Hum Cell. 2003. PMID: 12968785 - Adrenomedullin Function in Vascular Endothelial Cells: Insights from Genetic Mouse Models.
Karpinich NO, Hoopes SL, Kechele DO, Lenhart PM, Caron KM. Karpinich NO, et al. Curr Hypertens Rev. 2011 Dec;7(4):228-239. doi: 10.2174/157340211799304761. Curr Hypertens Rev. 2011. PMID: 22582036 Free PMC article.
References
- Dalgaard O Z. Acta Med Scand. 1957;328,Suppl.:1–255. - PubMed
- Grantham J J. Adv Intern Med. 1993;38:409–420. - PubMed
- Baert L. Kidney Int. 1978;13:519–525. - PubMed
- Gabow P A. N Eng J Med. 1993;329:332–342. - PubMed
- Huston J D, Torres V E, Sulivan P P, Offord K P, Wiebers D O. J Am Soc Nephrol. 1993;3:1871–1877. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases