Role for dynamin in late endosome dynamics and trafficking of the cation-independent mannose 6-phosphate receptor - PubMed (original) (raw)
Role for dynamin in late endosome dynamics and trafficking of the cation-independent mannose 6-phosphate receptor
P Nicoziani et al. Mol Biol Cell. 2000 Feb.
Free PMC article
Abstract
It is well established that dynamin is involved in clathrin-dependent endocytosis, but relatively little is known about possible intracellular functions of this GTPase. Using confocal imaging, we found that endogenous dynamin was associated with the plasma membrane, the trans-Golgi network, and a perinuclear cluster of cation-independent mannose 6-phosphate receptor (CI-MPR)-containing structures. By electron microscopy (EM), it was shown that these structures were late endosomes and that the endogenous dynamin was preferentially localized to tubulo-vesicular appendices on these late endosomes. Upon induction of the dominant-negative dynK44A mutant, confocal microscopy demonstrated a redistribution of the CI-MPR in mutant-expressing cells. Quantitative EM analysis of the ratio of CI-MPR to lysosome-associated membrane protein-1 in endosome profiles revealed a higher colocalization of the two markers in dynK44A-expressing cells than in control cells. Western blot analysis showed that dynK44A-expressing cells had an increased cellular procathepsin D content. Finally, EM revealed that in dynK44A-expressing cells, endosomal tubules containing CI-MPR were formed. These results are in contrast to recent reports that dynamin-2 is exclusively associated with endocytic structures at the plasma membrane. They suggest instead that endogenous dynamin also plays an important role in the molecular machinery behind the recycling of the CI-MPR from endosomes to the trans-Golgi network, and we propose that dynamin is required for the final scission of vesicles budding from endosome tubules.
Figures
Figure 1
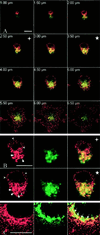
Distribution of endogenous dynamin in HeLa cells. HeLa dynK44A cells grown in the presence of tetracycline were fixed, permeabilized, and double labeled for dynamin (red in A–C) and CI-MPR (green in A and B) or for dynamin and TGN-38 (green in C). (A) Merged images obtained by confocal sectioning from the dorsal top to the ventral surface of a representative cell at 0.5-μm intervals. Note that although the dynamin signal derives in part from the plasma membrane, dynamin distinctly colocalizes with CI-MPR in the perinuclear region (yellow). (B) Split red, green, and merged channels of the 2.5-μm (+) and 3.5-μm (*) confocal section planes at higher magnification. Endogenous dynamin appears as both small dots (arrowheads) and larger, coarse dots (arrows), the latter colocalizing with the CI-MPR. (C) Dynamin also colocalizes to some extent with TGN-38 (arrows in the merged image). Bars, 10 μm.
Figure 2
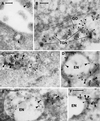
Localization of endogenous dynamin by immunogold labeling of ultracryosections. The localization of endogenous dynamin in HeLa dynK44A cells grown in the presence of tetracycline was determined by labeling of ultracryosections with the hudy-1 antibody (5-nm gold). Dynamin is localized to clathrin-coated pits at the cell surface (large arrowheads in A) and to the TGN adjacent to a Golgi stack (GO in B), where the membrane network characteristic of the TGN is outlined by arrowheads. Moreover, dynamin is localized to endosomes (EN) that contain internalized 20-nm cationized gold particles (large arrowheads in C) and CI-MPR (10-nm gold; large arrowheads in D–F). Note that dynamin (small arrowheads in C–F) is localized mainly to tubulo-vesicular extensions of the endosomes. Bar in A, 100 nm; bars in B–F, 200 nm.
Figure 3
Relative distribution of endogenous dynamin between the plasma membrane and the intracellular compartments. Ultracryosections from three different HeLa dynK44A cultures (at least two passages apart) grown in the presence of tetracycline were labeled with the hudy-1 antibody. Gold particles (5 nm) associated with or <100 nm away from the plasma membrane, including clathrin-coated pits (PM), Golgi stacks/TGN (Golgi), vacuolar and tubulo-vesicular endosomes (Endos.), and the cytosol, including unidentified cytosolic objects (Cytosol), were counted on randomly photographed EM pictures (magnification, ×84,000). A total of 8018 gold particles were scored. Values are means ± SE (n = 3).
Figure 4
Enhancement of mutant dynamin expression. (A) HeLa dynK44A cells were grown for 48 h without tetracycline (−tet) or for 72 h without tetracycline and in the presence of the transcriptional activator butyric acid (2 mM) for the last 24 h (−tet/+b.a.). The cells were then fixed, and double fluorescence labeling for dynamin (with the use of hudy-1) and actin was performed to determine the frequency of cells positive for mutant dynamin relative to cells positive for actin, which is present in all cells (mean ± SE, n = 3). (B) Representative Western blot of dynamin expression in HeLa dynK44A cells grown under the conditions described in A.
Figure 5
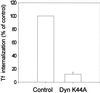
Expression of mutant dynamin inhibits endocytosis of transferrin. HeLa dynK44A cells were grown for 72 h with (Control) or without (Dyn K44A) tetracycline, in both cases in the presence of butyric acid for the last 24 h, and internalization of 125I-transferrin (Tf) was measured (mean ± SE, n = 3).
Figure 6
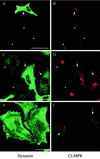
Expression of mutant dynamin causes redistribution of CI-MPR. HeLa dynK44A cells were grown without tetracycline for 48 h (A–D) or without tetracycline for 72 h and with butyric acid added for the last 24 h (E and F) and double-labeled for dynamin (green) and CI-MPR (red). Note that in the mutant dynamin–expressing cells (large arrows), the CI-MPR signal derives from more distinct and widespread structures than in cells without mutant expression (small arrows). The settings of the confocal microscope were adjusted individually in the two channels to make an optimal distinction between endogenous dynamin and mutant dynamin in each image, as well as an optimal CI-MPR signal. Bar in A, 50 μm; bars in C and E, 20 μm.
Figure 7
Representative electron micrographs of the patterns of MPR/Lamp-1 distribution as revealed by immunogold double labeling of ultracryosections. Based on the relative amount of gold labeling for CI-MPR and Lamp-1, endocytic structures were grouped into six types (type 1, MPR+/Lamp-1−; type 2, MPR/Lamp-1 3:1; type 3, MPR/Lamp-1 2:1; type 4, MPR/Lamp-1 1:2; type 5, MPR/Lamp-1 1:3; type 6, MPR−/Lamp-1+). Type 1 (only CI-MPR labeling) represents a classic endosome and type 6 (only Lamp-1 labeling) represents a classic lysosome. A–F show examples of types 1–6, respectively. CI-MPR was detected with 10-nm gold (small arrows in D–F), and Lamp-1 was detected with 15-nm gold (arrowheads in A–C). Bar, 200 nm.
Figure 8
Expression of mutant dynamin causes a redistribution of the CI-MPR to an endocytic compartment strongly positive for Lamp-1 but distinct from classic lysosomes. The frequency of types 1–6 endocytic structures in control cells and cells expressing mutant dynamin was compared based on immunogold double-labeling experiments and the extent of CI-MPR/Lamp-1 colocalization, as described in Figure 7 (bars, mean ± SE, n = 3). Black dots represent the distribution of CI-MPR among the compartments (mean ± SE, n = 3). The material analyzed includes 800 endocytic organelle profiles pooled from three independent experiments with control cells and 614 endocytic organelle profiles from three independent experiments with cells expressing mutant dynamin.
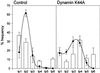
Figure 9
Cathepsin D maturation is perturbed by expression of mutant dynamin. (A) Expression of the lysosomal enzyme cathepsin D (32 kDa) and its precursor procathepsin D (49 kDa) was analyzed by Western blotting of cell lysates (5% of total lysates) and of medium collected from the last 24 h of culture (immunoprecipitates from 50% of medium). When cells were grown without tetracycline (−) in the medium for 48 h, there was a clear increase in cell-associated procathepsin D (left panel). However, even after 72 h without tetracycline and in the presence of butyric acid for the last 24 h, no change was found in the amount of secreted cathepsin into the medium (right panel). Small arrows, procathepsin D; large arrows, mature cathepsin. (B) Graph illustrating the increase in cellular procathepsin D caused by dynK44A expression. The graph is based on scanned blots from three experiments performed after 48 h without tetracycline (○) and six experiments performed after 72 h without tetracycline and in the presence of butyric acid for the last 24 h (●). For a statistical analysis, see text.
Figure 10
Expression of mutant dynamin causes endosome tubulation. (A and B) HRP-labeled endocytic structures in HeLa dynK44A cells grown in the presence of tetracycline. (C–E) HRP-labeled endocytic structures in the cells grown without tetracycline for 48 h. Cells were incubated with HRP for 30–50 min at 37°C. Note that more tubular endosomes were generated in the mutant-expressing cells. (F and G) Ultracryosections of the tubulated endosomes (small arrowheads) seen in cells expressing dynK44A. The sections were labeled for dynamin (5-nm gold) and CI-MPR (10-nm gold, large arrowheads). Bars in A–D, 1 μm; bars in E–G, 200 nm.
Similar articles
- Expression of mutant dynamin inhibits toxicity and transport of endocytosed ricin to the Golgi apparatus.
Llorente A, Rapak A, Schmid SL, van Deurs B, Sandvig K. Llorente A, et al. J Cell Biol. 1998 Feb 9;140(3):553-63. doi: 10.1083/jcb.140.3.553. J Cell Biol. 1998. PMID: 9456316 Free PMC article. - Endocytosed cation-independent mannose 6-phosphate receptor traffics via the endocytic recycling compartment en route to the trans-Golgi network and a subpopulation of late endosomes.
Lin SX, Mallet WG, Huang AY, Maxfield FR. Lin SX, et al. Mol Biol Cell. 2004 Feb;15(2):721-33. doi: 10.1091/mbc.e03-07-0497. Epub 2003 Oct 31. Mol Biol Cell. 2004. PMID: 14595110 Free PMC article. - Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles.
van Dam EM, Stoorvogel W. van Dam EM, et al. Mol Biol Cell. 2002 Jan;13(1):169-82. doi: 10.1091/mbc.01-07-0380. Mol Biol Cell. 2002. PMID: 11809831 Free PMC article. - Visualization of TGN-endosome trafficking in mammalian and Drosophila cells.
Kametaka S, Waguri S. Kametaka S, et al. Methods Enzymol. 2012;504:255-71. doi: 10.1016/B978-0-12-391857-4.00013-6. Methods Enzymol. 2012. PMID: 22264539 Review. - The dynamin family of mechanoenzymes: pinching in new places.
McNiven MA, Cao H, Pitts KR, Yoon Y. McNiven MA, et al. Trends Biochem Sci. 2000 Mar;25(3):115-20. doi: 10.1016/s0968-0004(99)01538-8. Trends Biochem Sci. 2000. PMID: 10694881 Review.
Cited by
- Inhibition of HIV-1 endocytosis allows lipid mixing at the plasma membrane, but not complete fusion.
de la Vega M, Marin M, Kondo N, Miyauchi K, Kim Y, Epand RF, Epand RM, Melikyan GB. de la Vega M, et al. Retrovirology. 2011 Dec 6;8:99. doi: 10.1186/1742-4690-8-99. Retrovirology. 2011. PMID: 22145853 Free PMC article. - Metastasis-suppressor NME1 controls the invasive switch of breast cancer by regulating MT1-MMP surface clearance.
Lodillinsky C, Fuhrmann L, Irondelle M, Pylypenko O, Li XY, Bonsang-Kitzis H, Reyal F, Vacher S, Calmel C, De Wever O, Bièche I, Lacombe ML, Eiján AM, Houdusse A, Vincent-Salomon A, Weiss SJ, Chavrier P, Boissan M. Lodillinsky C, et al. Oncogene. 2021 Jun;40(23):4019-4032. doi: 10.1038/s41388-021-01826-1. Epub 2021 May 19. Oncogene. 2021. PMID: 34012098 Free PMC article. - Dynamin regulates focal exocytosis in phagocytosing macrophages.
Di A, Nelson DJ, Bindokas V, Brown ME, Libunao F, Palfrey HC. Di A, et al. Mol Biol Cell. 2003 May;14(5):2016-28. doi: 10.1091/mbc.e02-09-0626. Epub 2003 Feb 21. Mol Biol Cell. 2003. PMID: 12802072 Free PMC article. - Vps10p cycles between the TGN and the late endosome via the plasma membrane in clathrin mutants.
Deloche O, Schekman RW. Deloche O, et al. Mol Biol Cell. 2002 Dec;13(12):4296-307. doi: 10.1091/mbc.02-07-0105. Mol Biol Cell. 2002. PMID: 12475953 Free PMC article. - Downregulation of the Na(+)- D-glucose cotransporter SGLT1 by protein RS1 (RSC1A1) is dependent on dynamin and protein kinase C.
Veyhl M, Wagner CA, Gorboulev V, Schmitt BM, Lang F, Koepsell H. Veyhl M, et al. J Membr Biol. 2003 Nov 1;196(1):71-81. doi: 10.1007/s00232-003-0626-y. J Membr Biol. 2003. PMID: 14724758
References
- Boleti H, Benmerah A, Ojcius DM, Cerf-Bensussan N, Dautry-Varsat A. Chlamydia infection of epithelial cells expressing dynamin and Eps15 mutants: clathrin-independent entry into cells and dynamin-dependent productive growth. J Cell Sci. 1999;112:1487–1496. - PubMed
- Bright NA, Reaves BJ, Mullock BM, Luzio PJ. Dense core lysosomes can fuse with late endosomes and are reformed from the resultant hybrid organelles. J Cell Sci. 1997;110:2027–2040. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous