Parallel actin bundles and their multiple actin-bundling proteins - PubMed (original) (raw)
Review
Parallel actin bundles and their multiple actin-bundling proteins
J R Bartles. Curr Opin Cell Biol. 2000 Feb.
Abstract
Parallel actin bundles are present in a diverse array of structures, where they are critical determinants of cellular shape and physiology. In the past 18 months, new findings have solidified the concept that parallel actin bundles are assembled in cells through the sequential action of multiple actin-bundling proteins and have begun to shed light on the roles played by the individual actin-bundling proteins.
Figures
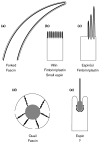
Figure 1. Schematic depictions of some cellular structures that contain parallel actin bundles in sectional view (not to scale) and a listing of their actin-bundling proteins
The parallel actin bundles are shown as thick black lines when revealed in longitudinal section (a--d) or as black dots when revealed in transverse section (e). The plasma membrane is shown as a thin black line. (a) Neurosensory bristle of Drosophila. These actin bundles can be up to 70 μm (microchaete) or 400 μm (macrochaete) in length. Although bristles and their actin bundles taper, microchaetes contain >500 filaments per bundle at their base [4,5,9**]. (b) Brush border microvilli. Depending on location, these actin bundles are typically 1--5 μm in length and contain 20--25 actin filaments per bundle [21]. (c) Hair cell stereocilia. Depending on location, these actin bundles vary in length from 1--10 μm and can contain up to 900 actin filaments per bundle [34,35]. (d) Cytoplasmic actin bundles of Drosophila nurse cell. These actin bundles are composed of a linear series of relatively uniform bundle modules that are ∼3 μm in length, contain ∼25 actin filaments and appear to overlap like the units of an extension ladder [8,29-31]. The nurse cell nucleus is shown in gray, and the openings in the plasma membrane represent the ring canals. (e) Sertoli cell-spermatid ectoplasmic specialization. These actin bundles form a layer that is only 5-7 actin filaments in width that appears to wrap around the invagination of the Sertoli cell plasma membrane that is in contact with the acrosomal region of the head of an elongating spermatid [23,44,45]. The spermatid is shown in gray.
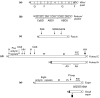
Figure 2. Schematic stick-figure diagrams of the actin-bundling proteins implicated in the assembly of the cellular structures shown in Figure 1, highlighting the relative positions and sizes of selected elements of primary sequence with structural or functional significance (roughly to scale)
Villin/Quail: Villin and quail are composed of six copies of an ∼15-kDa repeat characteristic of the members of the gelsolin family of actin-binding proteins (numbered 1-6), which account for the severing activity of villin observed in the presence of relatively high concentrations of calcium ion, and an ∼9-kDa actin-binding “headpiece” domain (HP), which is required for actin-bundling activity noted at lower concentrations of calcium ion. The actin monomer (G) or actin filament (F) binding portions of the molecule are shown. Fimbrin/Plastin: The fimbrins/plastins are members of the calponin-homology superfamily of actin-binding proteins and contains two ∼27-kD actin-binding domains (ABD1 and ABD2), each of which in turn contains two calponin homology domains (CH1,CH1′, CH2 and CH2′, respectively), and an N-terminal calcium ion-binding domain (CaBD) composed of two EF hand motifs. There are three highly related isoforms of fimbrin/plastin in mammals that are expressed in a cell-type specific manner. The I-isoform is present in the mature brush border microvillus. (Those interested in learning about the three-dimensional structures of the domains of villin and fimbrin/plastin should consult [3].) Fascin: Homologs of fascin have been detected in species ranging from echinoderms to humans. The positions of two point mutations that reduce the actin-bundling activity of Drosophila fascin (S289 and G409) and the position of the protein kinase C-mediated phosphorylation that inhibits the actin-bundling activity of human fascin (S39) are shown. Forked C and Forked A: The forked gene encodes at least 6 different transcripts, the products of different sites of transcriptional initiation and differential splicing. Shown here are the proteins encoded by one of the major transcripts, forked A, the ∼70-kDa isoform that has been examined in cell transfection experiments, and forked C, one of the largest transcripts, which is predicted to encode an ∼155-kDa protein that contains the maximum number of N-terminal ankyrin repeats and is included for the sake of comparion with espin (see below). An N-terminal peptide that is specific to forked A has been shaded, but regions of alignment between forked C and forked A that have not been shaded are identical. Also noted for the forked proteins are: the 66-amino acid forked/espin homology domain (F/E), proline-rich peptides (Pr), peptides containing an unusually high percentage of hydrophobic amino acids (H), peptides rich in Gln and His encoded by CAX trinucleotide repeats (CAX), and the peptides encoded by exons A3 and A5. Espin and Small Espin: The ∼110-kDa espin and the ∼30-kDa small espin are splice-isoforms that are expressed in a cell type-specific fashion. Peptides that are unique to small espin have been shaded, but regions of alignment between espin and small espin that are not shaded are identical. Also noted for the espins are: their shared 116-amino acid C-terminal actin-bundling module (shared ABM), which includes the 66-amino acid forked/espin homology domain (F/E); a potential P-loop, which is present in both isoforms; and the two proline-rich peptides (Pr) and 8 N-terminal ankyrin repeats that are present in espin, but not in small espin.
Similar articles
- Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo.
Loomis PA, Zheng L, Sekerková G, Changyaleket B, Mugnaini E, Bartles JR. Loomis PA, et al. J Cell Biol. 2003 Dec 8;163(5):1045-55. doi: 10.1083/jcb.200309093. Epub 2003 Dec 1. J Cell Biol. 2003. PMID: 14657236 Free PMC article. - Espin contains an additional actin-binding site in its N terminus and is a major actin-bundling protein of the Sertoli cell-spermatid ectoplasmic specialization junctional plaque.
Chen B, Li A, Wang D, Wang M, Zheng L, Bartles JR. Chen B, et al. Mol Biol Cell. 1999 Dec;10(12):4327-39. doi: 10.1091/mbc.10.12.4327. Mol Biol Cell. 1999. PMID: 10588661 Free PMC article. - Espins and the actin cytoskeleton of hair cell stereocilia and sensory cell microvilli.
Sekerková G, Zheng L, Loomis PA, Mugnaini E, Bartles JR. Sekerková G, et al. Cell Mol Life Sci. 2006 Oct;63(19-20):2329-41. doi: 10.1007/s00018-006-6148-x. Cell Mol Life Sci. 2006. PMID: 16909209 Free PMC article. Review. - Fascin 2b is a component of stereocilia that lengthens actin-based protrusions.
Chou SW, Hwang P, Gomez G, Fernando CA, West MC, Pollock LM, Lin-Jones J, Burnside B, McDermott BM Jr. Chou SW, et al. PLoS One. 2011;6(4):e14807. doi: 10.1371/journal.pone.0014807. Epub 2011 Apr 26. PLoS One. 2011. PMID: 21625653 Free PMC article. - Plastins regulate ectoplasmic specialization via its actin bundling activity on microfilaments in the rat testis.
Li N, Wong CK, Cheng CY. Li N, et al. Asian J Androl. 2016 Sep-Oct;18(5):716-22. doi: 10.4103/1008-682X.166583. Asian J Androl. 2016. PMID: 26608945 Free PMC article. Review.
Cited by
- Actin bundle architecture and mechanics regulate myosin II force generation.
Weirich KL, Stam S, Munro E, Gardel ML. Weirich KL, et al. Biophys J. 2021 May 18;120(10):1957-1970. doi: 10.1016/j.bpj.2021.03.026. Epub 2021 Mar 31. Biophys J. 2021. PMID: 33798565 Free PMC article. - α-Actinin and fimbrin cooperate with myosin II to organize actomyosin bundles during contractile-ring assembly.
Laporte D, Ojkic N, Vavylonis D, Wu JQ. Laporte D, et al. Mol Biol Cell. 2012 Aug;23(16):3094-110. doi: 10.1091/mbc.E12-02-0123. Epub 2012 Jun 27. Mol Biol Cell. 2012. PMID: 22740629 Free PMC article. - Roles of a fimbrin and an alpha-actinin-like protein in fission yeast cell polarization and cytokinesis.
Wu JQ, Bähler J, Pringle JR. Wu JQ, et al. Mol Biol Cell. 2001 Apr;12(4):1061-77. doi: 10.1091/mbc.12.4.1061. Mol Biol Cell. 2001. PMID: 11294907 Free PMC article. - A myosin motor that selects bundled actin for motility.
Nagy S, Ricca BL, Norstrom MF, Courson DS, Brawley CM, Smithback PA, Rock RS. Nagy S, et al. Proc Natl Acad Sci U S A. 2008 Jul 15;105(28):9616-20. doi: 10.1073/pnas.0802592105. Epub 2008 Jul 3. Proc Natl Acad Sci U S A. 2008. PMID: 18599451 Free PMC article. - Model for Bundling of Keratin Intermediate Filaments.
Haimov E, Windoffer R, Leube RE, Urbakh M, Kozlov MM. Haimov E, et al. Biophys J. 2020 Jul 7;119(1):65-74. doi: 10.1016/j.bpj.2020.05.024. Epub 2020 Jun 2. Biophys J. 2020. PMID: 32533940 Free PMC article.
References
- Matsudaira P. Modular organization of actin crosslinking proteins. Trends Biochem Sci. 1991;16:87–92. - PubMed
- Furukawa R, Fechheimer M. The structure, function, and assembly of actin filament bundles. Int Rev Cytol. 1997;175:29–90. - PubMed
- Puius YA, Mahoney NM, Almo SC. The modular structure of actin-regulatory proteins. Curr Opin Cell Biol. 1998;10:23–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K02 HD01210/HD/NICHD NIH HHS/United States
- K02 HD001210/HD/NICHD NIH HHS/United States
- R01 HD035280/HD/NICHD NIH HHS/United States
- R01 DA035280/DA/NIDA NIH HHS/United States
- R01 HD35280/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous