The yeast nuclear pore complex: composition, architecture, and transport mechanism - PubMed (original) (raw)
The yeast nuclear pore complex: composition, architecture, and transport mechanism
M P Rout et al. J Cell Biol. 2000.
Abstract
An understanding of how the nuclear pore complex (NPC) mediates nucleocytoplasmic exchange requires a comprehensive inventory of the molecular components of the NPC and a knowledge of how each component contributes to the overall structure of this large molecular translocation machine. Therefore, we have taken a comprehensive approach to classify all components of the yeast NPC (nucleoporins). This involved identifying all the proteins present in a highly enriched NPC fraction, determining which of these proteins were nucleoporins, and localizing each nucleoporin within the NPC. Using these data, we present a map of the molecular architecture of the yeast NPC and provide evidence for a Brownian affinity gating mechanism for nucleocytoplasmic transport.
Figures
Figure 1
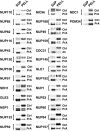
Identification of proteins in the highly enriched NPC fraction. Proteins in the highly enriched NPC fraction were separated by hydroxyapatite HPLC and SDS-PAGE. The number above each lane indicates the corresponding fraction number. Proteins were visualized with Coomassie blue; the approximate molecular mass of proteins as estimated from standards shown on the left side. Bands analyzed by MALDI-TOF mass spectrometry are indicated by the adjacent numbers. The proteins identified in each band are shown in the top panel. Proteins known to directly associate with the NPC are colored blue, while proteins believed not to be NPC-associated are colored red. On the top left are listed proteins not found in this separation but identified by MS/MS of reverse phase HPLC-separated peptides (RP/HPLC) or peptide microsequencing (PROT SEQ).
Figure 2
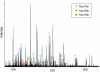
Identification of proteins in a gel band by mass spectrometry. MALDI-TOF mass spectrum of the peptides produced by in-gel trypsin digestion of band no. 217 (molecular mass of ∼100 kD, fraction 35). The peak masses were used to identify proteins present in the band with the protein identification algorithm ProFound. The dominant protein component was Nup120p, which was identified with a probability 5 × 1022 higher than the next most probable protein. The peaks arising from Nup120p were subtracted from the spectrum and a new search was initiated, identifying Kap120p with a probability 3 × 109 higher than the next most probable protein. Finally, the peaks arising from Kap120p were subtracted from the spectrum and a new search was initiated identifying Pdr6p/Kap122p with a probability 1 × 108 higher than the next most probable protein. Colored markers indicate which peaks arise from each of the three identified proteins. The peaks labeled T arise from trypsin self-digestion. Unlabeled peaks likely originate from modified or incompletely digested peptides in Nup120p, Kap120p, and Kap122p, or from additional proteins.
Figure 3
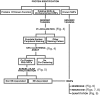
Flow chart outlining our experimental strategy for protein classification.
Figure 5
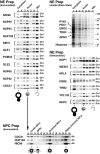
Relative distribution of tagged proteins in subcellular fractions. (Top right) Cells from the Nup42p Flu-tagged strain were fractionated and the proteins from each fraction were separated by SDS-PAGE and visualized by amido black. Lanes 1–4 were loaded at one cell equivalent and lanes 5–7 were loaded at three cell equivalents. Fraction 1 contains cytoplasmic material, 2 and 3 contain membranes, and 4 contains mainly nuclei. The nuclei were subjected to a second round of fractionation to obtain the two chromatin fractions 5 and 6, and fraction 7 enriched in NEs. The majority of proteins, including the indicated abundant cytoplasmic markers (Pyk1p, Pdc1p, Eno2p, Tdh3p, and Gpm1p) identified by mass spectrometry did not coenrich with the nuclei or the NEs. The histone triplet at ∼14 kD coenriched with nuclei but not NEs. (Top left) Proteins that cofractionate with NEs. Fractions from the same enrichment procedure for various tagged strains were probed for the internal standard Pom152p (Ctrl) and the protein of interest, mostly through a protein A tag (PrA). In a few cases, a FLU tag (Flu) or a monospecific antibody (Ab) was used. As expected, known nups coenriched with the NE-containing fractions, as did the newly identified Nup60p, Nup192p, and Pom34p. Seh1p, Gle1p, Gle2p, and Nup42p/Rip1p also coenriched with NEs, securing the classification of these proteins as nups. (Middle right) Proteins that do not cofractionate with NEs. Mex67p coenriched mainly with nuclei and partially with NEs, but a significant amount remained in the nucleoplasm in agreement with its recent classification as an important new mRNA export factor (Katahira et al. 1999). Similarly, both Pdr6p/Kap122p and Ypl125p/Kap120p showed a partial association with the highly enriched NEs during fractionation which, taken together with the immunofluorescence microscopy data, confirmed them as karyopherins. Neither Yrb2p nor Nup2p, which are closely related to each other at the primary sequence level, cofractionated with the NPC-containing fractions. Yrb2p is now known not to be a nup (see Fig. 4), but the significant amount of Nup2p remaining with the NE fraction points to a strong association with the NPCs. As Npl4p did not cofractionate with NPCs (and showed no NPC association by immunofluorescence microscopy; Fig. 4), we concluded that it is not a strongly bound component of the NPC. (Bottom) Nuclei were separated into fractions containing spindle pole bodies (SPBs), crude NPCs, enriched NPCs, and highly enriched NPCs; fraction numbers are as previously described (Rout and Kilmartin 1990). Cdc31p, which coenriched with the Pom152p control in the NE preparation, also cofractionated with Nic96p and Nup159p, two previously known nucleoporins, in the highly enriched NPC preparation, which allowed us to characterize Cdc31p as a component of the NPC. However, unlike the controls, significant amounts of Cdc31p were found in the SPB fraction. Cdc31p had given SPB staining plus some peripheral nuclear signal by immunofluorescence microscopy (Biggins and Rose 1994). Thus, Cdc31p appears to be present in both NPCs and SPBs, behaving in a similar fashion to Ndc1p (Chial et al. 1998).
Figure 4
Localization of PrA-tagged chimeras by immunofluorescence microscopy. Four images are shown for each protein. Scale bar is shown top right. The left pair shows the tagged protein localization (PROTEIN), while the right pair shows the corresponding positions of the nuclei in the same cells (DNA). The larger images illustrate the tag localization in otherwise wildtype cells, while the smaller images show localization in a nup120Δ strain in which the NPCs cluster to large discrete patches on the NE. NPC-associated proteins exhibit punctate staining around the nucleus in the Wildtype strain and cocluster with the NPCs in the Clustering strain. A sample of these is shown in the top box. This includes Gle1p and Gle2p, which had not been defined as nups. We also identified a typical NPC staining pattern for three previously uncharacterized ORFs: yar002w, yjl039c, and ylr018c. Further characterization of these three proteins demonstrated that they are indeed nups and that Ylr018p is an integral membrane protein (Fig. 5 and Fig. 6). In keeping with standard nomenclature, we term these proteins, Nup60p, Nup192p and Pom34p, respectively. Nup192p is the previously described 170-kD NPC-associated protein identified by peptide sequencing (Aitchison et al. 1995a). It has also recently been described as a potential nup by others (Kosova et al. 1999). Examples of proteins that do not display the punctate staining pattern, but still colocalize with the NPCs (Non-Punctate Rim, Co-Clustering) are shown in the lower left box, and include putative transport factors that partially associate with the NPC such as the karyopherin homologues Pdr6p and Ypl125p. Proteins that do not localize to the nuclear periphery and do not co-cluster with the NPCs (Non-Punctate Rim, Non-Clustering) are shown in the lower right box. The FG motif protein Yrb2p was found primarily in the nucleoplasm in agreement with recent studies (Noguchi et al. 1997; Taura et al. 1997). Although we did not find Npl4p in the highly enriched NPC fraction, it has been reported to be a possible nup (DeHoratius and Silver 1996). We detected this protein throughout the cytoplasm and observed no association with the NPC. The Npl4p-PrA chimera was functional, because although deletion of the NPL4 gene is lethal, we saw no growth defects in the haploid-tagged strain.
Figure 6
Ndc1p, Pom152p, and Pom34p are integral membrane proteins in the NPC. The NE fraction for each strain was extracted to obtain a supernatant fraction (SUP) and a pellet containing the integral membrane proteins (PELL). For each extraction, these fractions were probed for the protein of interest (PrA, Ab), the control transmembrane nup Pom152p (Ctrl), and known peripheral nups (not shown).
Figure 7
Localization of the tagged nucleoporins by immunoelectron microscopy. To estimate the position of the tagged nups within the NPC, we immunolabeled NEs from each protein A–tagged strain using a gold-conjugated antibody to visualize the tag. We selected labeled NPC images that had no obvious signs of damage or occlusion, and whose nucleocytoplasmic orientation could be unambiguously determined (Nehrbass et al. 1996). We chose only those NPCs sectioned perpendicular to the NE plane with a clearly visible double membrane. We selected a radius of 100 nm around the estimated center of each NPC as an excision limit and then created an aligned superimposed montage using 20 of the resulting excised NPC images (Strambio-de-Castillia et al. 1999). Scale bars are graduated in 10-nm intervals, with the horizontal bar centered on the cylindrical axis of the NPC and the vertical bar centered on the plane of NPC pseudo-mirror symmetry. The major features in each montage are diagrammed schematically on the lower right (C, cytoplasm; N, nucleoplasm). Montages from extraction conditions with the highest degree of specific labeling (averages ranging between 3–10 gold particles/NPC) are shown. They are arranged into those showing approximately symmetric labeling on both sides of the NPC, those with labeling biased towards one face, and those localized exclusively either to the cytoplasmic face or the nucleoplasmic face. NUP145N and NUP145C, NH2-terminal and COOH-terminal tagged fragments respectively of Nup145p (in all other figures and tables NUP145 refers only to the COOH-terminal tagged fragment).
Figure 8
Plot of the position of nucleoporins in the NPC. Statistical analysis of the distribution of the gold particles in each montage allows determination of the position of the proteins relative to the NPC cylindrical axis (R) and mirror plane of pseudosymmetry (Z). The plotted circle size was arbitrarily chosen for the sake of clarity. A mask for a cross-section of the yeast NPC and pore membrane is shown schematically to scale in light gray. We have highlighted the FG nups, the majority of which were found on both sides of the NPC (green), and a few that were found towards the periphery and exclusively on the nuclear side (blue) or the cytoplasmic side of the NPC (red). Most of the non-FG nups (dark gray) were found on both sides. The integral membrane protein Pom34p (purple) was close to the membrane, as were the inferred positions of Pom152p and Ndc1p (purple stripes).
Figure 9
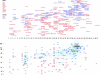
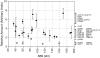
Relative abundance of nucleoporins in the NPC. Each data point represents the ratio of the signal from the tagged nucleoporin to the signal for the internal standard (Table ), which are proportional to the relative quantity of each protein in the NPC, averaged from at least two independent measurements for each nup (each generated from four different ratio measurements). The brackets indicate clusters of relative abundance containing the nups indicated at the right. Nups found exclusively on one side of the NPC are shown as open diamonds.
Figure 10
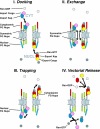
The Brownian affinity gate model. (I) The narrow bore of the central tube ensures that macromolecules that do not bind to nups find in hard to diffuse across the NPC, and are thus largely excluded; the diffusive movement of the filamentous nups may contribute to this exclusion. (II) Macromolecules that bind to nups increase their residence time at the entrance of the central tube, and so their diffusion across the NPC is greatly facilitated.
Figure 11
Diagram of NPC illustrating a model for nucleocytoplasmic transport. See Discussion for details.
Similar articles
- Comparative spatial localization of protein-A-tagged and authentic yeast nuclear pore complex proteins by immunogold electron microscopy.
Fahrenkrog B, Aris JP, Hurt EC, Panté N, Aebi U. Fahrenkrog B, et al. J Struct Biol. 2000 Apr;129(2-3):295-305. doi: 10.1006/jsbi.2000.4223. J Struct Biol. 2000. PMID: 10806080 - The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88.
Fornerod M, van Deursen J, van Baal S, Reynolds A, Davis D, Murti KG, Fransen J, Grosveld G. Fornerod M, et al. EMBO J. 1997 Feb 17;16(4):807-16. doi: 10.1093/emboj/16.4.807. EMBO J. 1997. PMID: 9049309 Free PMC article. - Molecular architecture of the yeast nuclear pore complex: localization of Nsp1p subcomplexes.
Fahrenkrog B, Hurt EC, Aebi U, Panté N. Fahrenkrog B, et al. J Cell Biol. 1998 Nov 2;143(3):577-88. doi: 10.1083/jcb.143.3.577. J Cell Biol. 1998. PMID: 9813081 Free PMC article. - Gatekeepers of the nucleus.
Wente SR. Wente SR. Science. 2000 May 26;288(5470):1374-7. doi: 10.1126/science.288.5470.1374. Science. 2000. PMID: 10827939 Review. - Genetic approaches to nuclear pore structure and function.
Doye V, Hurt EC. Doye V, et al. Trends Genet. 1995 Jun;11(6):235-41. doi: 10.1016/s0168-9525(00)89057-5. Trends Genet. 1995. PMID: 7638906 Review.
Cited by
- Nuclear Envelope and Nuclear Pore Complexes in Neurodegenerative Diseases-New Perspectives for Therapeutic Interventions.
Hachiya N, Sochocka M, Brzecka A, Shimizu T, Gąsiorowski K, Szczechowiak K, Leszek J. Hachiya N, et al. Mol Neurobiol. 2021 Mar;58(3):983-995. doi: 10.1007/s12035-020-02168-x. Epub 2020 Oct 17. Mol Neurobiol. 2021. PMID: 33067781 Free PMC article. Review. - The C-terminal domain of Nup93 is essential for assembly of the structural backbone of nuclear pore complexes.
Sachdev R, Sieverding C, Flötenmeyer M, Antonin W. Sachdev R, et al. Mol Biol Cell. 2012 Feb;23(4):740-9. doi: 10.1091/mbc.E11-09-0761. Epub 2011 Dec 14. Mol Biol Cell. 2012. PMID: 22171326 Free PMC article. - Promiscuous binding of Karyopherinβ1 modulates FG nucleoporin barrier function and expedites NTF2 transport kinetics.
Wagner RS, Kapinos LE, Marshall NJ, Stewart M, Lim RYH. Wagner RS, et al. Biophys J. 2015 Feb 17;108(4):918-927. doi: 10.1016/j.bpj.2014.12.041. Biophys J. 2015. PMID: 25692596 Free PMC article. - Congenital Heart Disease Genetics Uncovers Context-Dependent Organization and Function of Nucleoporins at Cilia.
Del Viso F, Huang F, Myers J, Chalfant M, Zhang Y, Reza N, Bewersdorf J, Lusk CP, Khokha MK. Del Viso F, et al. Dev Cell. 2016 Sep 12;38(5):478-92. doi: 10.1016/j.devcel.2016.08.002. Epub 2016 Sep 1. Dev Cell. 2016. PMID: 27593162 Free PMC article. - Venezuelan equine Encephalitis virus capsid protein forms a tetrameric complex with CRM1 and importin alpha/beta that obstructs nuclear pore complex function.
Atasheva S, Fish A, Fornerod M, Frolova EI. Atasheva S, et al. J Virol. 2010 May;84(9):4158-71. doi: 10.1128/JVI.02554-09. Epub 2010 Feb 10. J Virol. 2010. PMID: 20147401 Free PMC article.
References
- Aitchison J.D., Rout M.P., Marelli M., Blobel G., Wozniak R.W. Two novel related yeast nucleoporins Nup170p and Nup157pcomplementation with the vertebrate homologue Nup155p and functional interactions with the yeast nuclear pore-membrane protein Pom152p J. Cell Biol. 131 1995. 1133 1148a - PMC - PubMed
- Aitchison J.D., Blobel G., Rout M.P. Kap104pa karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science. 1996;274:624–627. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases