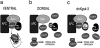Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification - PubMed (original) (raw)
Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification
G H Farr 3rd et al. J Cell Biol. 2000.
Abstract
Glycogen synthase kinase 3 (GSK-3) is a constitutively active kinase that negatively regulates its substrates, one of which is beta-catenin, a downstream effector of the Wnt signaling pathway that is required for dorsal-ventral axis specification in the Xenopus embryo. GSK-3 activity is regulated through the opposing activities of multiple proteins. Axin, GSK-3, and beta-catenin form a complex that promotes the GSK-3-mediated phosphorylation and subsequent degradation of beta-catenin. Adenomatous polyposis coli (APC) joins the complex and downregulates beta-catenin in mammalian cells, but its role in Xenopus is less clear. In contrast, GBP, which is required for axis formation in Xenopus, binds and inhibits GSK-3. We show here that GSK-3 binding protein (GBP) inhibits GSK-3, in part, by preventing Axin from binding GSK-3. Similarly, we present evidence that a dominant-negative GSK-3 mutant, which causes the same effects as GBP, keeps endogenous GSK-3 from binding to Axin. We show that GBP also functions by preventing the GSK-3-mediated phosphorylation of a protein substrate without eliminating its catalytic activity. Finally, we show that the previously demonstrated axis-inducing property of overexpressed APC is attributable to its ability to stabilize cytoplasmic beta-catenin levels, demonstrating that APC is impinging upon the canonical Wnt pathway in this model system. These results contribute to our growing understanding of how GSK-3 regulation in the early embryo leads to regional differences in beta-catenin levels and establishment of the dorsal axis.
Figures
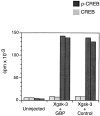
Figure 3
GBP does not inhibit Xgsk-3 phosphorylation of a peptide substrate. Embryos were injected with RNA encoding Xgsk-3-myc together with control RNA or GBP-FLAG RNA. After 3 h, proteins were extracted and immunoprecipitated with anti-FLAG (Xgsk-3 + GBP), anti-myc (Xgsk-3 + control), or both (uninjected) antibodies. The kinase activity of immune complexes was measured by phosphorus-32 incorporation into the GSK-3–specific substrate prephosphorylated CREB peptide (p-CREB; dark bars). The nonphosphorylated CREB peptide (CREB; light bars) is not a GSK-3 substrate and was used as a control. The activity of duplicate immune complexes is shown.
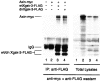
Figure 4
Dominant-negative Xgsk-3 binds Axin. Embryos were injected at the two- to eight-cell stage with 1 ng Axin-myc, 0.5 ng Xgsk-3-FLAG, and 0.5 ng dnXgsk-3-FLAG in the animal pole. Embryo extracts were precipitated with anti-FLAG antibody and detected by Western blotting (left panel). An aliquot of each sample taken before immunoprecipitation is shown in the right panel (Total Lysates). Lane numbers in the right panel refer to the same injections as shown above corresponding lane numbers in the left panel.
Figure 7
Model for GSK-3 regulation in the early Xenopus embryo. (a) On the ventral side of the embryo, GSK-3 is part of a functional degradation complex that includes APC and Axin. When in this complex, GSK-3 phosphorylates β-catenin, targeting it for degradation via the proteosome pathway. Under these conditions, dorsal genes are repressed. (b) On the dorsal side of the embryo, GSK-3 is excluded from the Axin–APC complex by GBP. In addition, GBP may prevent GSK-3 from phosphorylating its normal substrates by blocking access to the active site. β-Catenin accumulates and activates the transcription of dorsal genes. (c) The kinase-deficient dnXgsk-3 functions by binding Axin, thus, keeping endogenous Xgsk-3 from the degradation complex. As in the situation with GBP, β-catenin accumulates and activates the transcription of dorsal genes.
Similar articles
- Regulation of glycogen synthase kinase 3beta and downstream Wnt signaling by axin.
Hedgepeth CM, Deardorff MA, Rankin K, Klein PS. Hedgepeth CM, et al. Mol Cell Biol. 1999 Oct;19(10):7147-57. doi: 10.1128/MCB.19.10.7147. Mol Cell Biol. 1999. PMID: 10490650 Free PMC article. - Axil, a member of the Axin family, interacts with both glycogen synthase kinase 3beta and beta-catenin and inhibits axis formation of Xenopus embryos.
Yamamoto H, Kishida S, Uochi T, Ikeda S, Koyama S, Asashima M, Kikuchi A. Yamamoto H, et al. Mol Cell Biol. 1998 May;18(5):2867-75. doi: 10.1128/MCB.18.5.2867. Mol Cell Biol. 1998. PMID: 9566905 Free PMC article. - Relationship of vegetal cortical dorsal factors in the Xenopus egg with the Wnt/beta-catenin signaling pathway.
Marikawa Y, Elinson RP. Marikawa Y, et al. Mech Dev. 1999 Dec;89(1-2):93-102. doi: 10.1016/s0925-4773(99)00210-5. Mech Dev. 1999. PMID: 10559484 - Modulation of Wnt signaling by Axin and Axil.
Kikuchi A. Kikuchi A. Cytokine Growth Factor Rev. 1999 Sep-Dec;10(3-4):255-65. doi: 10.1016/s1359-6101(99)00017-9. Cytokine Growth Factor Rev. 1999. PMID: 10647780 Review. - New steps in the Wnt/beta-catenin signal transduction pathway.
Sakanaka C, Sun TQ, Williams LT. Sakanaka C, et al. Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review.
Cited by
- The links between axin and carcinogenesis.
Salahshor S, Woodgett JR. Salahshor S, et al. J Clin Pathol. 2005 Mar;58(3):225-36. doi: 10.1136/jcp.2003.009506. J Clin Pathol. 2005. PMID: 15735151 Free PMC article. Review. - Direct, activating interaction between glycogen synthase kinase-3beta and p53 after DNA damage.
Watcharasit P, Bijur GN, Zmijewski JW, Song L, Zmijewska A, Chen X, Johnson GV, Jope RS. Watcharasit P, et al. Proc Natl Acad Sci U S A. 2002 Jun 11;99(12):7951-5. doi: 10.1073/pnas.122062299. Epub 2002 Jun 4. Proc Natl Acad Sci U S A. 2002. PMID: 12048243 Free PMC article. - The pseudoreceptor BMP and activin membrane-bound inhibitor positively modulates Wnt/beta-catenin signaling.
Lin Z, Gao C, Ning Y, He X, Wu W, Chen YG. Lin Z, et al. J Biol Chem. 2008 Nov 28;283(48):33053-8. doi: 10.1074/jbc.M804039200. Epub 2008 Oct 6. J Biol Chem. 2008. PMID: 18838381 Free PMC article. - The maternally localized RNA fatvg is required for cortical rotation and germ cell formation.
Chan AP, Kloc M, Larabell CA, LeGros M, Etkin LD. Chan AP, et al. Mech Dev. 2007 May;124(5):350-63. doi: 10.1016/j.mod.2007.02.001. Epub 2007 Feb 21. Mech Dev. 2007. PMID: 17376659 Free PMC article. - Lithium regulates keratinocyte proliferation via glycogen synthase kinase 3 and NFAT2 (nuclear factor of activated T cells 2).
Hampton PJ, Jans R, Flockhart RJ, Parker G, Reynolds NJ. Hampton PJ, et al. J Cell Physiol. 2012 Apr;227(4):1529-37. doi: 10.1002/jcp.22872. J Cell Physiol. 2012. PMID: 21678407 Free PMC article.
References
- Behrens J., Jerchow B.A., Wurtele M., Grimm J., Asbrand C., Wirtz R., Kuhl M., Wedlich D., Birchmeier W. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK-3β. Science. 1998;280:596–599. - PubMed
- Behrens J., von Kries J.P., Kühl M., Bruhn L., Wedlich D., Grosschedl R., Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
- Bhanot P., Brink M., Samos C., Hsieh J., Wang Y., Macke J., Andrew D., Nathans J., Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. - PubMed
- Bienz M. APCthe plot thickens. Curr. Opin. Genet. Dev. 1999;9:595–603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HD007183/HD/NICHD NIH HHS/United States
- T32 GM007270/GM/NIGMS NIH HHS/United States
- R01 HD027262/HD/NICHD NIH HHS/United States
- T32GM07270/GM/NIGMS NIH HHS/United States
- HD27262/HD/NICHD NIH HHS/United States
- T32-HD07183/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials