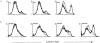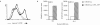Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells - PubMed (original) (raw)
Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells
U Yrlid et al. J Exp Med. 2000.
Abstract
Salmonella typhimurium is a gram-negative bacterium that survives and replicates inside vacuolar compartments of macrophages. Infection of macrophages with S. typhimurium grown under conditions allowing expression of the type III secretion system results in apoptotic death of the infected cells. Here, we show that infection of bone marrow-derived macrophages (MPhi) with wild-type S. typhimurium 14028 results in presentation of epitopes derived from a bacteria-encoded antigen on major histocompatibility complex (MHC) class I and MHC class II molecules after internalization of apoptotic MPhi by bystander dendritic cells (DCs). In contrast, infection of MPhi with the phoP constitutive mutant strain CS022, which does not induce apoptosis in infected MPhi, does not result in presentation of a bacteria-derived antigen by bystander DCs unless the infected MPhi are induced to undergo apoptosis by treatment with lipopolysaccharide and ATP. DCs appear to be unique in their ability to present antigens derived from MPhi induced to undergo apoptosis by Salmonella, as bystander MPhi are not capable of presenting the bacteria-derived antigen despite the fact that they efficiently internalize the apoptotic cells. These data suggest that apoptosis induction by bacterial infection of MPhi may not be a quiescent death that allows the bacteria to escape recognition by the immune system, but rather may contribute to an antimicrobial immune response upon engulfment by bystander DCs.
Figures
Figure 1
Logarithmically growing S. typhimurium 14028 induces apoptosis in MΦ, whereas the phoP c mutant CS022 or stationary phase 14028 does not. (a) MΦ were coincubated with increasing bacteria to MΦ ratios (5:1, 15:1, or 50:1) for 2 h. Residual bacteria were washed away, and the cells were incubated for an additional 2 h in medium with gentamycin (100 μg/ml). The cells were stained with Annexin V–FITC and PI and were analyzed in a flow cytometer. Histograms show 3 × 104 gated PI− cells plotted against cell number of MΦ coincubated with 14028s (thick line), CS022s (dotted line), or medium (thin line). (b) MΦ were coincubated with either logarithmically growing 14028r (thick line) or stationary phase (plate-grown) 14028r (dotted line) at a bacteria to MΦ ratio of 15:1, or were incubated in medium alone (thin line). At the times indicated in the histograms, infected cells were stained and analyzed as described in a. The induction of apoptosis in the MΦ was confirmed by FACS® analysis or detection of high molecular weight DNA fragments in at least three independent experiments.
Figure 2
Viable bystander DCs acquire antigenic material from MΦ induced to undergo apoptosis by infection with wild-type S. typhimurium. (a and c) OVA peptide presentation on MHC-I (Kb) and (d and f) MHC-II (I-Ab) by bystander DCs quantitated using the OVA(257–264)/Kb– or the OVA(265–277)/I-Ab–specific T cell hybridoma CD8OVA or OT4H.2D5, respectively. MΦ (H-2d) were coincubated for 90 min with either stationary phase (stat) 14028r, logarithmically growing (log) 14028r, or logarithmically growing CS022r expressing Crl-OVA as indicated. After washing and addition of gentamycin, live (+ DC) or paraformaldehyde-fixed (+ fixed DC) (H-2b) or no DCs (− DC) and T hybridoma cells were added as indicated. (b and e) The CD8OVA (b) or OT4H.2D5 (e) response to live or fixed DCs (H-2b) loaded with 1 nM OVA(257–264) or 100 μM OVA(265–280) peptide, respectively. Counts per minute in wells where bystander DCs were omitted or where DCs or MΦ were incubated in medium only along with the appropriate T cell hybridoma were typically 250–2,500. The results are representative of three to five independent experiments.
Figure 3
DCs process residual 14028 and CS022 expressing Crl-OVA for OVA peptide presentation on MHC-I and MHC-II equally well. Logarithmically growing (OD600 = 1.3–1.5) 14028r Crl-OVA or CS022r Crl-OVA bacteria were treated with 100 μg/ml of gentamycin (a and b), heat killed (HK) at 65°C (c and d), or were left untreated (e and f) before coincubation with DCs for 90 min. After several washes, OVA(257–264)/Kb (a, c, and e) and OVA(265–277)/I-Ab (b, d, and f) were quantitated using CD8OVA and OT4H.2D5 T hybridoma cells, respectively. The results are representative of three independent experiments.
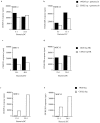
Figure 4
Chemical induction of apoptosis in MΦ infected with CS022 results in MHC-I and MHC-II presentation of bacteria-derived antigen by bystander DCs. (a) Histogram of flow cytometry analysis of Annexin V–FITC binding to 3 × 104 PI− MΦ that have phagocytosed logarithmically growing (log) CS022r Crl-OVA (dotted line) or CS022r Crl-OVA and were subsequently treated with LPS and ATP (thick line) or that were incubated in medium alone (thin line). Flow cytometry analysis was carried out 20 h after the apoptosis induction. (b) OVA(257–264)/Kb and OVA(265–277)/I-Ab presentation on MHC-I and MHC-II by bystander DCs (H-2b) after coincubation with MΦ (H-2d) previously coincubated with logarithmically growing CS022r Crl-OVA and left untreated or treated with LPS plus ATP as indicated. OVA(257–264)/Kb and OVA(265–277)/I-Ab presentation was quantitated using CD8OVA and OT4H.2D5 T hybridoma cells, respectively. (c) Viability of MΦ from wild-type and caspase 1−/− mice after coincubation with logarithmically growing 14028r Crl-OVA for 90 min measured as exclusion of PI staining. (d) MΦ derived from C57BL/6 or caspase 1−/− mice were coincubated with logarithmically growing 14028r Crl-OVA or medium only for 90 min. After washing and addition of gentamycin, DCs were added for 24 h. The DCs were then MACS-purified using anti-CD11c magnetic beads, and presentation of OVA(265–277) on MHC-II by the DCs was quantitated by addition of OT4H.2D5 T hybridoma cells to the MACS-purified DCs.
Figure 4
Chemical induction of apoptosis in MΦ infected with CS022 results in MHC-I and MHC-II presentation of bacteria-derived antigen by bystander DCs. (a) Histogram of flow cytometry analysis of Annexin V–FITC binding to 3 × 104 PI− MΦ that have phagocytosed logarithmically growing (log) CS022r Crl-OVA (dotted line) or CS022r Crl-OVA and were subsequently treated with LPS and ATP (thick line) or that were incubated in medium alone (thin line). Flow cytometry analysis was carried out 20 h after the apoptosis induction. (b) OVA(257–264)/Kb and OVA(265–277)/I-Ab presentation on MHC-I and MHC-II by bystander DCs (H-2b) after coincubation with MΦ (H-2d) previously coincubated with logarithmically growing CS022r Crl-OVA and left untreated or treated with LPS plus ATP as indicated. OVA(257–264)/Kb and OVA(265–277)/I-Ab presentation was quantitated using CD8OVA and OT4H.2D5 T hybridoma cells, respectively. (c) Viability of MΦ from wild-type and caspase 1−/− mice after coincubation with logarithmically growing 14028r Crl-OVA for 90 min measured as exclusion of PI staining. (d) MΦ derived from C57BL/6 or caspase 1−/− mice were coincubated with logarithmically growing 14028r Crl-OVA or medium only for 90 min. After washing and addition of gentamycin, DCs were added for 24 h. The DCs were then MACS-purified using anti-CD11c magnetic beads, and presentation of OVA(265–277) on MHC-II by the DCs was quantitated by addition of OT4H.2D5 T hybridoma cells to the MACS-purified DCs.
Figure 5
Both MΦ and DCs engulf apoptotic material by a process that requires cytoskeletal rearrangement despite the fact that OVA(257–264) presentation on MHC-I is restricted to DCs. OVA(257–264) presentation on MHC-I by bystander DCs (H-2b) (a) or MΦ (H-2b) (b) after coincubation with MΦ (H-2d) that previously phagocytosed logarithmically growing 14028r Crl-OVA or CS022r Crl-OVA. After 90 min of coincubation with bacteria, cells were washed, gentamycin was added, and OVA(257–264) presentation by added bystander cells was quantitated using CD8OVA T hybridoma cells. The response of MΦ (H-2d) and bystander cells incubated in medium only is also shown. (c) MΦ (H-2d) were coincubated with logarithmically growing 14028r Crl-OVA, CS022r Crl-OVA, or were incubated in medium only for 90 min. After washing and addition of gentamycin, DCs were added in the absence or presence of CCD (+ CCD) for 20 h. The DCs were then MACS-purified using anti-CD11c magnetic beads, and presentation of OVA(265–277) on MHC-II by the DCs was quantitated by addition of OT4H.2D5 T hybridoma cells to the MACS-purified DCs. The response of MΦ (H-2d) and bystander DCs incubated in medium only is also shown. (d) MΦ dyed red were either incubated in medium only or were coincubated with logarithmically growing 14028r or CS022r as indicated above the dot plots. These red MΦ were then coincubated with DCs (top) or MΦ (bottom) dyed green. This coincubation was done in the absence or presence (+ CCD) of CCD as indicated. Flow cytometry analysis was then performed on 3 × 104 cells. The percentage of double positive cells among green cells is indicated in each dot plot.
Similar articles
- Salmonella infection of bone marrow-derived macrophages and dendritic cells: influence on antigen presentation and initiating an immune response.
Yrlid U, Svensson M, Johansson C, Wick MJ. Yrlid U, et al. FEMS Immunol Med Microbiol. 2000 Apr;27(4):313-20. doi: 10.1111/j.1574-695X.2000.tb01445.x. FEMS Immunol Med Microbiol. 2000. PMID: 10727887 Review. - Bacterial antigen delivery systems: phagocytic processing of bacterial antigens for MHC-I and MHC-II presentation to T cells.
Svensson M, Pfeifer J, Stockinger B, Wick MJ. Svensson M, et al. Behring Inst Mitt. 1997 Feb;(98):197-211. Behring Inst Mitt. 1997. PMID: 9382741 Review. - Differential response of bovine monocyte-derived macrophages and dendritic cells to infection with Salmonella typhimurium in a low-dose model in vitro.
Norimatsu M, Harris J, Chance V, Dougan G, Howard CJ, Villarreal-Ramos B. Norimatsu M, et al. Immunology. 2003 Jan;108(1):55-61. doi: 10.1046/j.1365-2567.2003.01557.x. Immunology. 2003. PMID: 12519303 Free PMC article. - Salmonella enterica serovar typhimurium-induced maturation of bone marrow-derived dendritic cells.
Svensson M, Johansson C, Wick MJ. Svensson M, et al. Infect Immun. 2000 Nov;68(11):6311-20. doi: 10.1128/IAI.68.11.6311-6320.2000. Infect Immun. 2000. PMID: 11035740 Free PMC article. - Entry and survival of Salmonella typhimurium in dendritic cells and presentation of recombinant antigens do not require macrophage-specific virulence factors.
Niedergang F, Sirard JC, Blanc CT, Kraehenbuhl JP. Niedergang F, et al. Proc Natl Acad Sci U S A. 2000 Dec 19;97(26):14650-5. doi: 10.1073/pnas.97.26.14650. Proc Natl Acad Sci U S A. 2000. PMID: 11121065 Free PMC article.
Cited by
- Exploiting bacteria for cancer immunotherapy.
Kwon SY, Thi-Thu Ngo H, Son J, Hong Y, Min JJ. Kwon SY, et al. Nat Rev Clin Oncol. 2024 Aug;21(8):569-589. doi: 10.1038/s41571-024-00908-9. Epub 2024 Jun 5. Nat Rev Clin Oncol. 2024. PMID: 38840029 Review. - Efferocytosis in dendritic cells: an overlooked immunoregulatory process.
Ma Y, Jiang T, Zhu X, Xu Y, Wan K, Zhang T, Xie M. Ma Y, et al. Front Immunol. 2024 May 21;15:1415573. doi: 10.3389/fimmu.2024.1415573. eCollection 2024. Front Immunol. 2024. PMID: 38835772 Free PMC article. Review. - Efferocytosis and Its Role in Inflammatory Disorders.
Ge Y, Huang M, Yao YM. Ge Y, et al. Front Cell Dev Biol. 2022 Feb 25;10:839248. doi: 10.3389/fcell.2022.839248. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35281078 Free PMC article. Review. - Salmonella Infantis Delays the Death of Infected Epithelial Cells to Aggravate Bacterial Load by Intermittent Phosphorylation of Akt With SopB.
Chu BX, Li YN, Liu N, Yuan LX, Chen SY, Zhu YH, Wang JF. Chu BX, et al. Front Immunol. 2021 Nov 5;12:757909. doi: 10.3389/fimmu.2021.757909. eCollection 2021. Front Immunol. 2021. PMID: 34804044 Free PMC article. - Apoptotic Bodies: Mechanism of Formation, Isolation and Functional Relevance.
Santavanond JP, Rutter SF, Atkin-Smith GK, Poon IKH. Santavanond JP, et al. Subcell Biochem. 2021;97:61-88. doi: 10.1007/978-3-030-67171-6_4. Subcell Biochem. 2021. PMID: 33779914
References
- Neutra M.R., Frey A., Kraehenbuhl J.-P. Epithelial M cellsgateways for mucosal infection and immunization. Cell. 1996;86:345–348. - PubMed
- Jones B.D., Falkow S. Salmonellosishost immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 1996;14:533–561. - PubMed
- Neutra M.R., Pringault E., Kraehenbuhl J.-P. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu. Rev. Immunol. 1996;14:275–300. - PubMed
- Foster J.W., Spector M.P. How Salmonella survive against the odds. Annu. Rev. Microbiol. 1995;49:145–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials