ATR disruption leads to chromosomal fragmentation and early embryonic lethality - PubMed (original) (raw)
Comparative Study
. 2000 Feb 15;14(4):397-402.
Affiliations
- PMID: 10691732
- PMCID: PMC316378
Comparative Study
ATR disruption leads to chromosomal fragmentation and early embryonic lethality
E J Brown et al. Genes Dev. 2000.
Abstract
Although a small decrease in survival and increase in tumor incidence was observed in ATR(+/-) mice, ATR(-/-) embryos die early in development, subsequent to the blastocyst stage and prior to 7.5 days p.c. In culture, ATR(-/-) blastocysts cells continue to cycle into mitosis for 2 days but subsequently fail to expand and die of caspase-dependent apoptosis. Importantly, caspase-independent chromosome breaks are observed in ATR(-/-) cells prior to widespread apoptosis, implying that apoptosis is caused by a loss of genomic integrity. These data show that ATR is essential for early embryonic development and must function in processes other than regulation of p53.
Figures
Figure 1
Targeted disruption of ATR. (A) A schematic of the wild-type locus, targeting vector, and recombined locus are shown. In the targeting vector and recombined locus, the neomycin selection cassette replaces the first three coding exons of ATR, including the translation initiation codon (exon 1). Homologous recombination of the targeting vector into the ATR locus introduces an _Eco_RV site, shortening a 33-kb wild-type _Eco_RV fragment to 20 kb. (B) Southern blot of DNA from ES cell colonies confirms the projected truncation of the _Eco_RV fragment by homologous recombination. (Lanes 1,3) _Eco_RV-digested DNA from wild-type ES cells, (lanes 2,4) ES cells with a single recombined ATR allele. (C) DNA samples from wild-type (lanes 1,3) and ATR+/− (lanes 2,4) ES cells were subjected to PCR to confirm genotyping as performed by Southern analysis. PCR products derived from wild-type and disrupted alleles are 216 and 590 bp, respectively. (D) Northern blot of poly (A)+ mRNA from ATR+/+ and ATR+/− MEFs. Embryos used to generate MEFs (passage 2) were isolated from _ATR_-disrupted mouse lines 1 and 2 (see Materials and Methods). Autoradiographic detection of ATR, FRAP, and β-actin transcripts are shown.
Figure 2
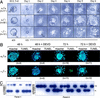
Deletion of ATR leads to apoptotic death of early embryonic cells. (A) Day 3.5 p.c. blastocysts were isolated from ATR+/− intercrosses and were cultured in 96-well plates for 6 days. ICMs and TGC are indicated. (B) TUNEL staining of ATR+/− and _ATR_−/− blastocysts cultured in the presence or absence of Z-DEVD-FMK. Blastocysts isolated from ATR+/− intercrosses were cultured in Terasaki-style microwell plates (Nunc) for 48 and 72 hr. Z-DEVD-FMK (200 μ
m
) or vehicle was added after 24 hr of culture (for 48- and 72-hr time points) and added again after 48 hr (72-hr time point). Fluorescein (TUNEL) and Hoechst fluorescent images are shown. (C) Examples of PCR genotyping of blastocysts cultured as described in A and B are shown in panels 1 and 2, respectively.
Figure 3
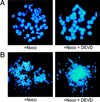
Chromosomal fragmentation in _ATR_−/− cells at day 2 in culture. Chromosome fragmentation was observed ranging from few (A) to many (B) breaks. This range of fragmentation was observed similarly in _ATR_−/− blastocysts cultured in the presence or absence of 200 μ
m
Z-DEVD-FMK added after 24 hr of culture.
References
- Aladjem MI, Spike BT, Rodewald LW, Hope TJ, Klemm M, Jaenisch R, Wahl GM. ES cells do not activate p53-dependent stress responses and undergo p53- independent apoptosis in response to DNA damage. Curr Biol. 1998;8:145–155. - PubMed
- Barlow C, Eckhaus MA, Schaffer AA, Wynshaw-Boris A. Atm haploinsufficiency results in increased sensitivity to sublethal doses of ionizing radiation in mice. Nat Genet. 1999;21:359–360. - PubMed
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. - PubMed
- Canman CE, Wolff AC, Chen CY, Fornace AJ, Jr, Kastan MB. The p53-dependent G1 cell cycle checkpoint pathway and ataxia-telangiectasia. Cancer Res. 1994;54:5054–5058. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous