Mammalian hepatocyte differentiation requires the transcription factor HNF-4alpha - PubMed (original) (raw)
. 2000 Feb 15;14(4):464-74.
Affiliations
- PMID: 10691738
- PMCID: PMC316377
Mammalian hepatocyte differentiation requires the transcription factor HNF-4alpha
J Li et al. Genes Dev. 2000.
Abstract
HNF-4alpha is a transcription factor of the nuclear hormone receptor family that is expressed in the hepatic diverticulum at the onset of liver development. Mouse embryos lacking HNF-4alpha fail to complete gastrulation due to dysfunction of the visceral endoderm. This early embryonic lethality has so far prevented any analyses of the contribution of HNF-4alpha toward liver development and hepatocyte differentiation. However, we have shown that complementation of HNF-4alpha(-/-) embryos with a tetraploid embryo-derived wild-type visceral endoderm rescues this early developmental arrest and allows HNF-4alpha(-/-) embryos to proceed normally through midgestation stages of development. Examination of these rescued embryos revealed that HNF-4alpha was dispensable for specification and early development of the liver. However, HNF-4alpha(-/-) fetal livers failed to express a large array of genes whose expression in differentiated hepatocytes is essential for a functional hepatic parenchyma, including genes encoding several apolipoproteins, metabolic proteins, and serum factors. In addition, we have demonstrated that HNF-4alpha is essential for expression of the transcription factors HNF-1alpha and PXR within the fetal liver. We therefore conclude that HNF-4alpha is both essential for hepatocyte differentiation during mammalian liver development and also crucial for metabolic regulation and liver function.
Figures
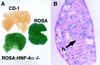
Figure 1
Hepatoblasts derived from _HNF-4α_−/− ES cells contribute toward the developing liver. (A) Fetal livers were isolated from wild-type (CD-1), ROSA26 (ROSA), or chimeric (ROSA: _HNF-4α_−/−) embryos at E11.5 and stained for β-galactosidase expression. (B) Photomicrograph showing a 5 μ
m
section through the chimeric fetal liver shown in A. The section was counterstained with eosin to show _HNF-4α_−/− hepatoblasts (h).
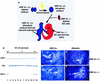
Figure 2
Hepatoblasts are present in embryos generated from _HNF-4α_−/− ES cells by tetraploid aggregation. (A) Embryos were generated from _HNF-4α_−/− ES cells (red) by aggregating the cells with HNF-4α+/+ embryos (blue) made tetraploid by electrofusion. Embryos continued their development in utero after transfer of the aggregates to psuedopregnant surrogate mothers. Fetal tissues generated by this procedure arise from _HNF-4α_−/− ES cells; the extraembryonic tissues, including the visceral endoderm, are tetraploid embryo derived. The presence of HNF-4α+/+ visceral endoderm allows _HNF-4α_−/− embryos to proceed through midgestation stages of development. (B) The genotype of embryos generated from HNF-4α+/+ (lane 1), HNF-4α+/− (lanes 11,12), and _HNF-4α_−/− ES cells (lanes 2_–_10) could be determined by PCR using primers which specifically amplified DNA fragments from the HPRT, bacterial neo, or HNF-4α (HNF-4) genes. (C_–_F) Dark-field micrographs showing in situ hybridization analyses that were performed on 5 μ
m
paraffin sections of E10.5 embryos generated from HNF-4α+/− (C,D) and _HNF-4α_−/− (E,F) ES cells. In all embryos, HNF-3α mRNAs (C,E) could be detected in gut endoderm (e) and hepatoblasts (h), whereas albumin mRNAs (D,F) were detected only in hepatoblasts.
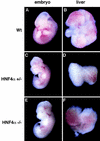
Figure 3
Whole mount micrographs showing wild-type CD-1, HNF-4α+/−, and _HNF-4α_−/− ES cell derived fetuses and livers after 12 days gestation. Embryos were removed and photographed under a dissecting microscope at 15× magnification. Livers were dissected and photographed at 70× magnification.
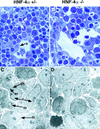
Figure 4
Light micrographs and electron micrographs comparing HNF-4α+/− and _HNF-4α_−/− fetal livers. (A,B) Sections (1 μ
m
) through livers from _HNF-4α_−/− or HNF-4α+/− ES cell-derived mice at E12. Sections were stained with toluidine blue and photographed using a 100× objective. Both HNF-4α+/− and _HNF-4α_−/− livers contained hepatoblasts (h), nucleated fetal blood cells (b), and endothelial cells (e) surrounding sinusoidal spaces. (C,D) Electron micrographs of the same fetal livers at 3000× magnification. Both dark (dh) and light (lh) hepatoblasts can be identified in HNF-4α+/− and _HNF-4α_−/− livers. The light hepatoblasts are believed to be in a less differentiated state containing a high nuclear (n) to cytoplasmic ratio and few organelles. In contrast, the more differentiated dark hepatoblasts have a relatively small nucleus and contain many organelles including endoplasmic reticulum (er) and mitochondria (m).
Figure 5
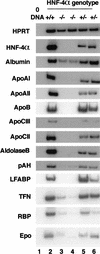
HNF-4_α_ is essential for normal hepatocyte differentiation. Steady-state mRNA levels from several genes normally expressed in differentiated hepatocytes was measured in livers isolated from HNF-4α+/+ (lane 2), HNF-4α+/− (lanes 5,6), and _HNF-4α_−/− (lanes 3,4) ES-cell-derived embryos at day 12 of gestation by RT–PCR. All samples contained comparable amounts of cDNA, as HPRT primers amplified a similar level of product in all cases. HPRT primers did not amplify product in the absence of cDNA (lane 1). apoAI, apoAII, apoB, apoCIII, apoCII, and albumin, aldolase B, pAH, LFABP, TFN, RBP, and Epo mRNA levels were similar in HNF-4+/+ and HNF-4+/− livers. In _HNF-4α_−/− livers the relative level of the same mRNAs was decreased dramatically and in some cases undetectable.
Figure 6
HNF-1α and PXR lie downstream of HNF-4α during hepatogenesis. Steady-state mRNA levels of several transcription factors acting in the fetal liver were measured in E12 HNF-4α+/+ (lane 2), HNF-4α+/− (lanes 5,6), and HNF-4α_−/− (lanes 3,4) livers by RT–PCR. Primers recognizing HNF-3α, -3_β, 3_γ, HNF-1β_, GATA-4, HNF-6, c/EBP-β and -γ, RAR-α, -β and -γ, RXR-α, and COUP-TFII all amplified similar levels of product in all fetal livers. However, HNF-1α and PXR steady-state mRNA levels (arrowed) were greatly decreased in _HNF-4α_−/− fetal livers.
Figure 7
Schematic depicting key points during hepatic development and the stage at which specific transcription factors first act. During gastrulation, at ∼7.0 days of gestation in the mouse, the definitive endoderm (yellow) is generated from the primitive ectoderm. Both ventral and dorsal aspects of the definitive embryonic endoderm express HNF-3 and are competent to adopt a hepatic fate. By the four- to six-somite stage a ventral portion of the embryonic endoderm (blue) lies close to the developing heart. During hepatic specification, FGFs (fgf's) from the developing cardiac mesoderm (red) induce the underlying endoderm to commit to a hepatic fate. In response to this signal the endodermal cells proliferate, expand into the surrounding septum transversum, and adopt a stellate morphology. At this point these cells are considered hepatoblasts. The hepatoblasts continue to proliferate and begin to differentiate to become mature hepatocyte in a process that requires HNF-4α.
Similar articles
- HNF-6 is expressed in endoderm derivatives and nervous system of the mouse embryo and participates to the cross-regulatory network of liver-enriched transcription factors.
Landry C, Clotman F, Hioki T, Oda H, Picard JJ, Lemaigre FP, Rousseau GG. Landry C, et al. Dev Biol. 1997 Dec 15;192(2):247-57. doi: 10.1006/dbio.1997.8757. Dev Biol. 1997. PMID: 9441665 - Role of the hepatocyte nuclear factor 4alpha in control of the pregnane X receptor during fetal liver development.
Kamiya A, Inoue Y, Gonzalez FJ. Kamiya A, et al. Hepatology. 2003 Jun;37(6):1375-84. doi: 10.1053/jhep.2003.50212. Hepatology. 2003. PMID: 12774017 - Regulatory mechanisms controlling human hepatocyte nuclear factor 4alpha gene expression.
Hatzis P, Talianidis I. Hatzis P, et al. Mol Cell Biol. 2001 Nov;21(21):7320-30. doi: 10.1128/MCB.21.21.7320-7330.2001. Mol Cell Biol. 2001. PMID: 11585914 Free PMC article. - Liver-enriched transcription factors and hepatocyte differentiation.
Cereghini S. Cereghini S. FASEB J. 1996 Feb;10(2):267-82. FASEB J. 1996. PMID: 8641560 Review. - Hepatocyte nuclear factor-4α, a multifunctional nuclear receptor associated with cardiovascular disease and cholesterol catabolism.
Tavares-Sanchez OL, Rodriguez C, Gortares-Moroyoqui P, Estrada MI. Tavares-Sanchez OL, et al. Int J Environ Health Res. 2015;25(2):126-39. doi: 10.1080/09603123.2014.915015. Epub 2014 May 22. Int J Environ Health Res. 2015. PMID: 24848804 Review.
Cited by
- Promoter Methylation Leads to Hepatocyte Nuclear Factor 4A Loss and Pancreatic Cancer Aggressiveness.
Hatziapostolou M, Koutsioumpa M, Zaitoun AM, Polytarchou C, Edderkaoui M, Mahurkar-Joshi S, Vadakekolathu J, D'Andrea D, Lay AR, Christodoulou N, Pham T, Yau TO, Vorvis C, Chatterji S, Pandol SJ, Poultsides GA, Dawson DW, Lobo DN, Iliopoulos D. Hatziapostolou M, et al. Gastro Hep Adv. 2024 Apr 24;3(5):687-702. doi: 10.1016/j.gastha.2024.04.005. eCollection 2024. Gastro Hep Adv. 2024. PMID: 39165427 Free PMC article. - Expression patterns of HNF4α, TTF-1, and SMARCA4 in lung adenocarcinomas: impacts on clinicopathological and genetic features.
Kawai H, Miura T, Kawamatsu N, Nakagawa T, Shiba-Ishii A, Yoshimoto T, Amano Y, Kihara A, Sakuma Y, Fujita K, Shibano T, Ishikawa S, Ushiku T, Fukayama M, Tsubochi H, Endo S, Hagiwara K, Matsubara D, Niki T. Kawai H, et al. Virchows Arch. 2024 May 6. doi: 10.1007/s00428-024-03816-6. Online ahead of print. Virchows Arch. 2024. PMID: 38710944 - Oligonucleotide therapies for nonalcoholic steatohepatitis.
Li S, Xiong F, Zhang S, Liu J, Gao G, Xie J, Wang Y. Li S, et al. Mol Ther Nucleic Acids. 2024 Mar 30;35(2):102184. doi: 10.1016/j.omtn.2024.102184. eCollection 2024 Jun 11. Mol Ther Nucleic Acids. 2024. PMID: 38665220 Free PMC article. Review. - txci-ATAC-seq: a massive-scale single-cell technique to profile chromatin accessibility.
Zhang H, Mulqueen RM, Iannuzo N, Farrera DO, Polverino F, Galligan JJ, Ledford JG, Adey AC, Cusanovich DA. Zhang H, et al. Genome Biol. 2024 Mar 22;25(1):78. doi: 10.1186/s13059-023-03150-1. Genome Biol. 2024. PMID: 38519979 Free PMC article. - Cellular reprogramming in vivo initiated by SOX4 pioneer factor activity.
Katsuda T, Sussman JH, Ito K, Katznelson A, Yuan S, Takenaka N, Li J, Merrell AJ, Cure H, Li Q, Rasool RU, Asangani IA, Zaret KS, Stanger BZ. Katsuda T, et al. Nat Commun. 2024 Feb 26;15(1):1761. doi: 10.1038/s41467-024-45939-z. Nat Commun. 2024. PMID: 38409161 Free PMC article.
References
- Ang S-L, Wierda A, Wong D, Stevens KA, Cascio S, Rossant J, Zaret KS. The formation and maintenance of the definitive endoderm lineage in the mouse: Involvement of HNF-3/forkhead proteins. Development. 1993;119:1301–1315. - PubMed
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. - PubMed
- Cardot P, Chambaz J, Kardassis D, Cladaras C, Zannis VI. Factors participating in the liver-specific expression of the human apolipoprotein A-II gene and their significance for transcription. Biochemistry. 1993;32:9080–9093. - PubMed
- Cascio S, Zaret KS. Hepatocyte differentiation initiates during endodermal-mesenchymal interactions prior to liver formation. Development. 1991;113:217–225. - PubMed
- Cereghini S. Liver-enriched transcription factors and hepatocyte differentiation. FASEB J. 1996;10:267–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases