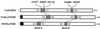TraG from RP4 and TraG and VirD4 from Ti plasmids confer relaxosome specificity to the conjugal transfer system of pTiC58 - PubMed (original) (raw)
TraG from RP4 and TraG and VirD4 from Ti plasmids confer relaxosome specificity to the conjugal transfer system of pTiC58
C M Hamilton et al. J Bacteriol. 2000 Mar.
Abstract
Plasmid conjugation systems are composed of two components, the DNA transfer and replication system, or Dtr, and the mating pair formation system, or Mpf. During conjugal transfer an essential factor, called the coupling protein, is thought to interface the Dtr, in the form of the relaxosome, with the Mpf, in the form of the mating bridge. These proteins, such as TraG from the IncP1 plasmid RP4 (TraG(RP4)) and TraG and VirD4 from the conjugal transfer and T-DNA transfer systems of Ti plasmids, are believed to dictate specificity of the interactions that can occur between different Dtr and Mpf components. The Ti plasmids of Agrobacterium tumefaciens do not mobilize vectors containing the oriT of RP4, but these IncP1 plasmid derivatives lack the trans-acting Dtr functions and TraG(RP4). A. tumefaciens donors transferred a chimeric plasmid that contains the oriT and Dtr genes of RP4 and the Mpf genes of pTiC58, indicating that the Ti plasmid mating bridge can interact with the RP4 relaxosome. However, the Ti plasmid did not mobilize transfer from an IncQ relaxosome. The Ti plasmid did mobilize such plasmids if TraG(RP4) was expressed in the donors. Mutations in traG(RP4) with defined effects on the RP4 transfer system exhibited similar phenotypes for Ti plasmid-mediated mobilization of the IncQ vector. When provided with VirD4, the tra system of pTiC58 mobilized plasmids from the IncQ relaxosome. However, neither TraG(RP4) nor VirD4 restored transfer to a traG mutant of the Ti plasmid. VirD4 also failed to complement a traG(RP4) mutant for transfer from the RP4 relaxosome or for RP4-mediated mobilization from the IncQ relaxosome. TraG(RP4)-mediated mobilization of the IncQ plasmid by pTiC58 did not inhibit Ti plasmid transfer, suggesting that the relaxosomes of the two plasmids do not compete for the same mating bridge. We conclude that TraG(RP4) and VirD4 couples the IncQ but not the Ti plasmid relaxosome to the Ti plasmid mating bridge. However, VirD4 cannot couple the IncP1 or the IncQ relaxosome to the RP4 mating bridge. These results support a model in which the coupling proteins specify the interactions between Dtr and Mpf components of mating systems.
Figures
FIG. 1
Structure and relatedness of coupling proteins from the RP4 and Ti plasmid transfer systems. The length of each protein in amino acid residues is indicated by the numbers at each end. Filled regions indicate amino acid sequences with properties of N-terminal secretion signals. The stippled and cross-hatched regions and their coordinates delimit the two motifs conserved among the members of the TraG family (19, 29). The amino acid substitution mutants of TraGRP4 are indicated by the single-letter designation for the wild-type residue followed by the position number and the mutant residue.
FIG. 2
Gene organization of the Dtr regions of the transfer systems from RP4 and pTiC58. The black-filled arrows represent genes coding for the coupling proteins of the three transfer systems. (A) tra locus of the Ti plasmid tra system. Locations of genes within the two tra operons, as well as the oriT sequence, are indicated by the large arrows. The flagstaff represents the site of the Tn_3_HoHo1 insertion located in traG of the mutant Ti plasmid pDCKI41. The diagonally hatched bar represents the _Eco_RI fragment from pTiC58 containing traG cloned in pDCE20. The small arrow represents the location and direction of transcription of the native TraR-dependent promoter responsible for expression of the traCDG operon. (B) Tra1 core region of RP4. The locations of the essential Dtr genes and the oriT are shown by large arrows. The diagonally hatched bar depicts the fragment containing the traG gene cloned in pBS141. The arrow represents the Tac promoter, provided by the vector, that drives expression of the gene. (C) virD operon of the Ti plasmid. The five genes of the virD operon are shown by the large arrows. The diagonally hatched bar depicts the fragment containing the virD4 gene cloned in pHL142. The arrow represents the lac promoter, provided by the vector, that drives expression of the gene.
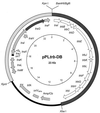
FIG. 3
Genetic structure of pPL_trb_-DB. This plasmid, based on the IncP1 vector pJB3 (thin black line) (5), contains the entire trb region from pTiC58 (open arrows) and the Tra1 core region from RP4 (light shaded arrows). traG, which codes for the RP4-coupling protein, is indicated by the dark shaded arrow. oriV, origin of vegetative plasmid replication; trfA, the gene coding for the replication initiation protein of RP4; Amp/Cb, the bla gene coding for resistance to ampicillin and carbenicillin. See Materials and Methods for details.
Similar articles
- Interaction of Bacteroides fragilis pLV22a relaxase and transfer DNA with Escherichia coli RP4-TraG coupling protein.
Thomas J, Hecht DW. Thomas J, et al. Mol Microbiol. 2007 Nov;66(4):948-60. doi: 10.1111/j.1365-2958.2007.05967.x. Epub 2007 Oct 5. Mol Microbiol. 2007. PMID: 17919288 Free PMC article. - The tra region of the nopaline-type Ti plasmid is a chimera with elements related to the transfer systems of RSF1010, RP4, and F.
Farrand SK, Hwang I, Cook DM. Farrand SK, et al. J Bacteriol. 1996 Jul;178(14):4233-47. doi: 10.1128/jb.178.14.4233-4247.1996. J Bacteriol. 1996. PMID: 8763953 Free PMC article. - Ti plasmid conjugation is independent of vir: reconstitution of the tra functions from pTiC58 as a binary system.
Cook DM, Li PL, Ruchaud F, Padden S, Farrand SK. Cook DM, et al. J Bacteriol. 1997 Feb;179(4):1291-7. doi: 10.1128/jb.179.4.1291-1297.1997. J Bacteriol. 1997. PMID: 9023214 Free PMC article. - The mating pair formation system of conjugative plasmids-A versatile secretion machinery for transfer of proteins and DNA.
Schröder G, Lanka E. Schröder G, et al. Plasmid. 2005 Jul;54(1):1-25. doi: 10.1016/j.plasmid.2005.02.001. Plasmid. 2005. PMID: 15907535 Review. - Analysis of the sequence and gene products of the transfer region of the F sex factor.
Frost LS, Ippen-Ihler K, Skurray RA. Frost LS, et al. Microbiol Rev. 1994 Jun;58(2):162-210. doi: 10.1128/mr.58.2.162-210.1994. Microbiol Rev. 1994. PMID: 7915817 Free PMC article. Review.
Cited by
- Interaction of Bacteroides fragilis pLV22a relaxase and transfer DNA with Escherichia coli RP4-TraG coupling protein.
Thomas J, Hecht DW. Thomas J, et al. Mol Microbiol. 2007 Nov;66(4):948-60. doi: 10.1111/j.1365-2958.2007.05967.x. Epub 2007 Oct 5. Mol Microbiol. 2007. PMID: 17919288 Free PMC article. - Detergent extraction identifies different VirB protein subassemblies of the type IV secretion machinery in the membranes of Agrobacterium tumefaciens.
Krall L, Wiedemann U, Unsin G, Weiss S, Domke N, Baron C. Krall L, et al. Proc Natl Acad Sci U S A. 2002 Aug 20;99(17):11405-10. doi: 10.1073/pnas.172390699. Epub 2002 Aug 12. Proc Natl Acad Sci U S A. 2002. PMID: 12177443 Free PMC article. - Transfer of R388 derivatives by a pathogenesis-associated type IV secretion system into both bacteria and human cells.
Fernández-González E, de Paz HD, Alperi A, Agúndez L, Faustmann M, Sangari FJ, Dehio C, Llosa M. Fernández-González E, et al. J Bacteriol. 2011 Nov;193(22):6257-65. doi: 10.1128/JB.05905-11. Epub 2011 Sep 9. J Bacteriol. 2011. PMID: 21908662 Free PMC article. - Transcriptome analysis revealed that a quorum sensing system regulates the transfer of the pAt megaplasmid in Agrobacterium tumefaciens.
Mhedbi-Hajri N, Yahiaoui N, Mondy S, Hue N, Pélissier F, Faure D, Dessaux Y. Mhedbi-Hajri N, et al. BMC Genomics. 2016 Aug 20;17:661. doi: 10.1186/s12864-016-3007-5. BMC Genomics. 2016. PMID: 27543103 Free PMC article. - Origin-of-transfer sequences facilitate mobilisation of non-conjugative antimicrobial-resistance plasmids in Staphylococcus aureus.
O'Brien FG, Yui Eto K, Murphy RJ, Fairhurst HM, Coombs GW, Grubb WB, Ramsay JP. O'Brien FG, et al. Nucleic Acids Res. 2015 Sep 18;43(16):7971-83. doi: 10.1093/nar/gkv755. Epub 2015 Aug 3. Nucleic Acids Res. 2015. PMID: 26243776 Free PMC article.
References
- Beijersbergen A, den Dulk-Ras A, Schilperoort R A, Hooykaas P J J. Conjugative transfer by the virulence system of Agrobacterium tumefaciens. Science. 1992;256:1324–1327. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous