The peripheral antinociceptive effect induced by morphine is associated with ATP-sensitive K(+) channels - PubMed (original) (raw)
The peripheral antinociceptive effect induced by morphine is associated with ATP-sensitive K(+) channels
A R Rodrigues et al. Br J Pharmacol. 2000 Jan.
Abstract
The effect of several K(+) channel blockers such as glibenclamide, tolbutamide, charybdotoxin (ChTX), apamin, tetraethylammonium (TEA), 4-aminopyridine (4-AP) and cesium on the peripheral antinociceptive effect of morphine was evaluated by the paw pressure test in Wistar rats. The intraplantar administration of a carrageenan suspension (250 microg) resulted in an acute inflammatory response and a decreased threshold to noxious pressure. Morphine administered locally into the paw (25, 50, 100 and 200 microg) elicited a dose-dependent antinociceptive effect which was demonstrated to be mediated by a peripheral site up to the 100 microg dose. The selective blockers of ATP-sensitive K(+) channels glibenclamide (20, 40 and 80 microg paw(-1)) and tolbutamide (40, 80 and 160 microg paw(-1)) antagonized the peripheral antinociception induced by morphine (100 microg paw(-1)). This effect was unaffected by ChTX (0. 5, 1.0 and 2.0 microg paw(-1)), a large conductance Ca(2+)-activated K(+) channel blocker, or by apamin (2.5, 5.0 and 10.0 microg paw(-1)), a selective blocker of a small conductance Ca(2+)-activated K(+) channel. Intraplantar administration of the non-specific K(+) channel blockers TEA (160, 320 and 640 microg), 4-AP (10, 50 and 100 microg) and cesium (125, 250 and 500 microg) also did not modify the peripheral antinociceptive effect of morphine. These results suggest that the peripheral antinociceptive effect of morphine may result from activation of ATP-sensitive K(+) channels, which may cause a hyperpolarization of peripheral terminals of primary afferents, leading to a decrease in action potential generation. In contrast, large conductance Ca(2+)-activated K(+) channels, small conductance Ca(2+)-activated K(+) channels as well as voltage-dependent K(+) channels appear not to be involved in this transduction pathway. British Journal of Pharmacology (2000) 129, 110 - 114
Figures
Figure 1
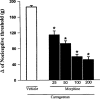
Effect of morphine on the nociceptive threshold in carrageenan-induced hyperalgesia in rats. Morphine (μg) was administered intraplantarly 2 h after the local administration of 100 μl of a carrageenan suspension (250 μg). Each column represents the mean±s.e.mean (_n_=10). *P<0.01 vs carrageenan+vehicle-injected control (Bonferroni's test).
Figure 2
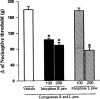
Exclusion of a central antinociceptive effect of morphine. Morphine (μg) was administered into the right (R) or left (L) paw 2 h after carrageenan administration into both hind paws. Each column represents the mean±s.e.mean (_n_=10). *P<0.01 vs carrageenan+ vehicle-injected control (Bonferroni's test).
Figure 3
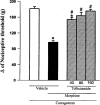
Antagonism induced by intraplantar administration of glibenclamide of the peripheral antinociception produced by morphine in hyperalgesic paws. Glibenclamide (μg) was administered 5 min before morphine (100 μg). Each column represents the mean±s.e.mean (_n_=5). * and # P<0.01 as compared to (Carrageenan+vehicles) and (Carrageenan+morphine+vehicle)- injected controls, respectively (Bonferroni's test).
Figure 4
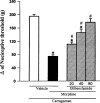
Antagonism induced by intraplantar administration of tolbutamide of the peripheral antinociception produced by morphine in hyperalgesic paws. Tolbutamide (μg) was administered 5 min before morphine (100 μg). Each column represents the mean±s.e.mean (_n_=5). * and # P<0.01 as compared to (Carrageenan+vehicles) and (Carrageenan+morphine+vehicle)-injected controls, respectively (Bonferroni's test).
Figure 5
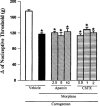
Effect of intraplantar administration of apamin and ChTX on the peripheral antinociception induced by morphine in hyperalgesic paws. Apamin and charybdotoxin (μg) were administered 45 min after morphine (100 μg). Each column represents the mean±s.e.mean (_n_=5). No statistically significant differences were detected between the groups treated with morphine+vehicle and morphine+apamin or ChTX in any case. *P<0.01 vs carrageenan+vehicle-injected control (Bonferroni's test).
Figure 6
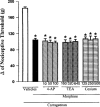
Effect of intraplantar administration of 4-AP, TEA and Cesium on the peripheral antinociception induced by morphine in hyperalgesic paws. 4-AP, TEA and Cesium (μg paw−1) were administered 45 min after morphine (100 μg paw−1). Each column represents the mean±s.e.mean (_n_=5). No statistically significant differences between the groups treated with carrageenan+morphine+vehicle and carrageenan+morphine+4-AP, TEA or Cesium were found in any case. *P<0.01 vs carrageenan+vehicle-injected control (Bonferroni's test).
Similar articles
- Participation of ATP-sensitive K+ channels in the peripheral antinociceptive effect of fentanyl in rats.
Rodrigues AR, Castro MS, Francischi JN, Perez AC, Duarte ID. Rodrigues AR, et al. Braz J Med Biol Res. 2005 Jan;38(1):91-7. doi: 10.1590/s0100-879x2005000100014. Epub 2005 Jan 18. Braz J Med Biol Res. 2005. PMID: 15665994 - Dibutyryl-cyclic GMP induces peripheral antinociception via activation of ATP-sensitive K(+) channels in the rat PGE2-induced hyperalgesic paw.
Soares AC, Duarte ID. Soares AC, et al. Br J Pharmacol. 2001 Sep;134(1):127-31. doi: 10.1038/sj.bjp.0704224. Br J Pharmacol. 2001. PMID: 11522604 Free PMC article. - The peripheral antinociceptive effect of resveratrol is associated with activation of potassium channels.
Granados-Soto V, Argüelles CF, Ortiz MI. Granados-Soto V, et al. Neuropharmacology. 2002 Oct;43(5):917-23. doi: 10.1016/s0028-3908(02)00130-2. Neuropharmacology. 2002. PMID: 12384177 - Bis-quinolinium cyclophanes: highly potent and selective non-peptidic blockers of the apamin-sensitive Ca2+-activated K+ channel.
Conejo-García A, Campos JM. Conejo-García A, et al. Curr Med Chem. 2008;15(13):1305-15. doi: 10.2174/092986708784534983. Curr Med Chem. 2008. PMID: 18537610 Review. - Intracellular signalling involved in activation of the volume-sensitive K+ current in Ehrlich ascites tumour cells.
Hoffmann EK, Hougaard C. Hoffmann EK, et al. Comp Biochem Physiol A Mol Integr Physiol. 2001 Oct;130(3):355-66. doi: 10.1016/s1095-6433(01)00419-6. Comp Biochem Physiol A Mol Integr Physiol. 2001. PMID: 11913449 Review.
Cited by
- Participation of the NO/cGMP/K+ATP pathway in the antinociception induced by Walker tumor bearing in rats.
Barbosa AL, Pinheiro CA, Oliveira GJ, Torres JN, Moraes MO, Ribeiro RA, Vale ML, Souza MH. Barbosa AL, et al. Braz J Med Biol Res. 2012 Jun;45(6):531-6. doi: 10.1590/s0100-879x2012007500047. Epub 2012 Mar 29. Braz J Med Biol Res. 2012. PMID: 22450376 Free PMC article. - Inhibition of acid-sensing ion channels by diminazene and APETx2 evoke partial and highly variable antihyperalgesia in a rat model of inflammatory pain.
Lee JYP, Saez NJ, Cristofori-Armstrong B, Anangi R, King GF, Smith MT, Rash LD. Lee JYP, et al. Br J Pharmacol. 2018 Jun;175(12):2204-2218. doi: 10.1111/bph.14089. Epub 2018 Jan 3. Br J Pharmacol. 2018. PMID: 29134638 Free PMC article. - Skin vasodilation and analgesic effect of a topical nitric oxide-releasing hydrogel.
Vercelino R, Cunha TM, Ferreira ES, Cunha FQ, Ferreira SH, de Oliveira MG. Vercelino R, et al. J Mater Sci Mater Med. 2013 Sep;24(9):2157-69. doi: 10.1007/s10856-013-4973-7. Epub 2013 Jun 12. J Mater Sci Mater Med. 2013. PMID: 23756965 - A Novel Peripheral Action of PICK1 Inhibition in Inflammatory Pain.
Jensen KL, Noes-Holt G, Sørensen AT, Madsen KL. Jensen KL, et al. Front Cell Neurosci. 2021 Dec 15;15:750902. doi: 10.3389/fncel.2021.750902. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34975407 Free PMC article. - Role of pertussis toxin-sensitive G-protein, K+ channels, and voltage-gated Ca2+ channels in the antinociceptive effect of inosine.
Macedo-Junior SJ, Nascimento FP, Luiz-Cerutti M, Santos AR. Macedo-Junior SJ, et al. Purinergic Signal. 2013 Mar;9(1):51-8. doi: 10.1007/s11302-012-9327-2. Epub 2012 Jul 18. Purinergic Signal. 2013. PMID: 22806273 Free PMC article.
References
- BABENKO A.P., AGUILAR-BRYAN L., BRYAN J. A view of Sur/KIR 6.X, KATP channels. Annu. Rev. Physiol. 1998;60:667–687. - PubMed
- COOK N.S., QUAST U. Potassium channel pharmacology Potassium channels: Structure, classification, function, and therapeutic potential 1990Chichester: Ellis Horwood Limited; 181–255.ed. Cook. N.S. pp
- DUARTE I.D.G., DOSSANTOS I.R., LORENZETTI B.B., FERREIRA S.H. Analgesia by direct antagonism of nociceptor sensitisation involves the arginine-nitric oxide-cGMP pathway. Eur. J. Pharmacol. 1992;217:225–227. - PubMed
- EDWARDS G., WESTON A.H. The pharmacology of ATP-sensitive potassium channels. Annu. Rev. Pharmacol. Toxicol. 1993;33:597–637. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous