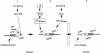Differential regulation of gene expression by insulin and IGF-1 receptors correlates with phosphorylation of a single amino acid residue in the forkhead transcription factor FKHR - PubMed (original) (raw)
Differential regulation of gene expression by insulin and IGF-1 receptors correlates with phosphorylation of a single amino acid residue in the forkhead transcription factor FKHR
J Nakae et al. EMBO J. 2000.
Abstract
The transcription factor FKHR is inhibited by phosphorylation in response to insulin and IGF-1 through Akt kinase. Here we show that FKHR phosphorylation in hepatocytes conforms to a hierarchical pattern in which phosphorylation of the Akt site at S(253), in the forkhead DNA binding domain, is a prerequisite for the phosphorylation of two additional potential Akt sites, T(24) and S(316). Using insulin receptor-deficient hepatocytes, we show that T(24) fails to be phosphorylated by IGF-1 receptors, suggesting that this residue is targeted by a kinase specifically activated by insulin receptors. Lack of T(24) phosphorylation is associated with the failure of IGF-1 to induce nuclear export of FKHR, and to inhibit expression of a reporter gene under the transcriptional control of the IGF binding protein-1 insulin response element. We propose that site-specific phosphorylation of FKHR is one of the mechanisms by which insulin and IGF-1 receptors exert different effects on gene expression.
Figures
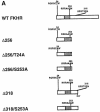
Fig. 1. Insulin-dependent phosphorylation of wild-type and mutant FKHR constructs in SV40-transformed murine hepatocytes. (A) Deletion constructs utilized in the present study. The consensus Akt sites are indicated in the single-letter amino acid code. The phosphorylated or mutant residue is underlined. The forkhead domain is represented by the shaded box. (B) Replacement of S253D partially restores insulin-dependent phosphorylation of FKHR. Normal mouse hepatocytes were transiently transfected with wild-type and mutant FKHR constructs, and insulin-induced phosphorylation was studied using 32P labeling. In some experiments, cells were treated with wortmannin prior to the addition of insulin (right hand panel). In the upper panel, following immunoprecipitation with anti-_c_–myc antibody and SDS–PAGE, proteins were transferred to nylon membranes for autoradiography. The filters were then re-probed with an anti-FKHR antiserum to normalize the protein content of each lane (lower panel). (C) T24 and S316 are phosphorylated in S253D mutant FKHR. Experiments were performed as indicated above, except that double and triple mutants were used, as shown at the top of each panel.
Fig. 1. Insulin-dependent phosphorylation of wild-type and mutant FKHR constructs in SV40-transformed murine hepatocytes. (A) Deletion constructs utilized in the present study. The consensus Akt sites are indicated in the single-letter amino acid code. The phosphorylated or mutant residue is underlined. The forkhead domain is represented by the shaded box. (B) Replacement of S253D partially restores insulin-dependent phosphorylation of FKHR. Normal mouse hepatocytes were transiently transfected with wild-type and mutant FKHR constructs, and insulin-induced phosphorylation was studied using 32P labeling. In some experiments, cells were treated with wortmannin prior to the addition of insulin (right hand panel). In the upper panel, following immunoprecipitation with anti-_c_–myc antibody and SDS–PAGE, proteins were transferred to nylon membranes for autoradiography. The filters were then re-probed with an anti-FKHR antiserum to normalize the protein content of each lane (lower panel). (C) T24 and S316 are phosphorylated in S253D mutant FKHR. Experiments were performed as indicated above, except that double and triple mutants were used, as shown at the top of each panel.
Fig. 1. Insulin-dependent phosphorylation of wild-type and mutant FKHR constructs in SV40-transformed murine hepatocytes. (A) Deletion constructs utilized in the present study. The consensus Akt sites are indicated in the single-letter amino acid code. The phosphorylated or mutant residue is underlined. The forkhead domain is represented by the shaded box. (B) Replacement of S253D partially restores insulin-dependent phosphorylation of FKHR. Normal mouse hepatocytes were transiently transfected with wild-type and mutant FKHR constructs, and insulin-induced phosphorylation was studied using 32P labeling. In some experiments, cells were treated with wortmannin prior to the addition of insulin (right hand panel). In the upper panel, following immunoprecipitation with anti-_c_–myc antibody and SDS–PAGE, proteins were transferred to nylon membranes for autoradiography. The filters were then re-probed with an anti-FKHR antiserum to normalize the protein content of each lane (lower panel). (C) T24 and S316 are phosphorylated in S253D mutant FKHR. Experiments were performed as indicated above, except that double and triple mutants were used, as shown at the top of each panel.
Fig. 2. The C-terminal half of FKHR contains negative regulatory elements for FKHR phosphorylation by insulin. Two truncated mutants were employed for these experiments, one encompassing amino acids 1–255 (Δ256) and one encompassing amino acids 1–317 (Δ318). Additional mutations were introduced as indicated at the top of each lane (see also Figure 1A). Phosphorylation experiments and quantification of the data were carried out as indicated in the legend to Figure 1.
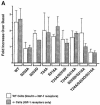
Fig. 3. (A) Different patterns of FKHR phosphorylation are mediated by insulin and IGF–1. Wild-type and mutant FKHR as indicated in Figure 1A were transfected into SV40-transformed hepatocytes derived from either normal mice (empty bars) or IR-deficient mice (full bars). Each experiment was repeated a minimum of three times. The data are expressed as the mean ± SD of the fold increase over basal phosphorylation levels in the absence of insulin (control hepatocytes) or IGF–1 (IR-deficient hepatocytes), and were quantitated using scanning densitometry and the NIH Image 1.60 software. The difference between the effect of insulin to phosphorylate the S253D/S316A mutant in WT and IR-deficient hepatocytes is statistically significant (P <0.05 analyzed by one-factor ANOVA). (B) T24 is not phosphorylated in response to IGF–1 treatment in IR-deficient hepatocytes. The mutants employed for these experiments are indicated at the top of each lane. A representative experiment is shown.
Fig. 3. (A) Different patterns of FKHR phosphorylation are mediated by insulin and IGF–1. Wild-type and mutant FKHR as indicated in Figure 1A were transfected into SV40-transformed hepatocytes derived from either normal mice (empty bars) or IR-deficient mice (full bars). Each experiment was repeated a minimum of three times. The data are expressed as the mean ± SD of the fold increase over basal phosphorylation levels in the absence of insulin (control hepatocytes) or IGF–1 (IR-deficient hepatocytes), and were quantitated using scanning densitometry and the NIH Image 1.60 software. The difference between the effect of insulin to phosphorylate the S253D/S316A mutant in WT and IR-deficient hepatocytes is statistically significant (P <0.05 analyzed by one-factor ANOVA). (B) T24 is not phosphorylated in response to IGF–1 treatment in IR-deficient hepatocytes. The mutants employed for these experiments are indicated at the top of each lane. A representative experiment is shown.
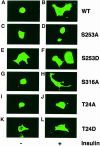
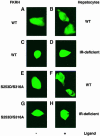
Fig. 4. Subcellular localization of phosphorylation site mutant FKHRs in insulin-treated SV40-transformed hepatocytes. Following transient transfection, hepatocytes were seeded into 4-well slide culture chambers and incubated in serum-free medium for 4 h prior to the addition of insulin. Myc epitope-tagged FKHR was visualized with anti-myc monoclonal antibody and FITC-conjugated anti-mouse IgG. (A and B) Wild-type FKHR; (C and D) S253A mutant; (E and F) S253D mutant; (G and H) S316A mutant; (I and J) T24A mutant; (K and L) T24D mutant.
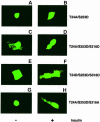
Fig. 5. Role of T24 in subcellular localization of FKHR. Experi- ments were performed as indicated in the legend to Figure 4. (A and B) T24A/S253D double mutant; (C and D) T24A/S253D/S316D triple mutant; (E and F) T24D/S253D/S316D triple mutant; (G and H) T24A/S253D/S316A triple mutant.
Fig. 6. Subcellular localization of an S253D/S316A double mutant FKHR in control and IR-deficient hepatocytes. Experiments were performed as indicated in the legend to Figure 4, except that IR-deficient hepatocytes were treated with IGF–1 to induce nuclear export of FKHR. (A and B) Nuclear exclusion of wild-type FKHR in insulin-treated SV40-transformed hepatocytes from normal mice; (C and D) nuclear exclusion of wild-type FKHR in IGF–1-treated IR-deficient hepatocytes; (E and F) nuclear exclusion of S253D/S316A FKHR in insulin-treated control hepatocytes; (G and H) nuclear exclusion of S253D/S316A FKHR in IGF–1-treated IR-deficient hepatocytes.
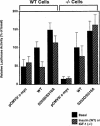
Fig. 7. Expression of an IGFBP–1/luciferase reporter gene in hepatocytes co-transfected with wild-type and S253D/S316A mutant FKHR. Control and IR-deficient hepatocytes were transfected with wild-type or mutant FKHR in addition to the IGFBP–1/luciferase reporter gene (p925GL3) (Ooi et al., 1992). Plasmid pRSV-β–galactosidase was used as an internal control of transfection efficiency (Ooi et al., 1992). After transfection, β–galactosidase and luciferase activity were measured in the same sample as described in Materials and methods. The data represent the mean ± SD from five independent experiments.
Fig. 8. Proposed model for multisite regulation of FKHR function. Under conditions in which insulin signaling is inactive, FKHR localizes to the nucleus. Following insulin stimulation, Akt translocates to the nucleus and phosphorylates S253 in the forkhead domain of FKHR. This primes FKHR for further phosphorylation of T24 and S316. T24 phosphorylation is effected by an IR-specific kinase and is required for FKHR nuclear export. S316 phosphorylation is effected by both IR and IGF–1R-stimulated kinases, and is not a requisite for FKHR nuclear export. Once FKHR is phosphorylated on T24 and exported to the cytoplasm, pT24 has the potential to bind 14-3-3 and cause cytoplasmic retention of FKHR (Brunet et al., 1999). The T24 and S316 kinases are also PIP3 dependent, like Akt. Nevertheless, it is unlikely that the T24 kinase is Akt, because Akt is activated to the same extent in control and IR-deficient hepatocytes (B.C.Park et al., 1999), and T24 fails to be phosphorylated in IR-deficient hepatocytes.
Similar articles
- Insulin regulation of gene expression through the forkhead transcription factor Foxo1 (Fkhr) requires kinases distinct from Akt.
Nakae J, Kitamura T, Ogawa W, Kasuga M, Accili D. Nakae J, et al. Biochemistry. 2001 Oct 2;40(39):11768-76. doi: 10.1021/bi015532m. Biochemistry. 2001. PMID: 11570877 - Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targetting.
Rena G, Prescott AR, Guo S, Cohen P, Unterman TG. Rena G, et al. Biochem J. 2001 Mar 15;354(Pt 3):605-12. doi: 10.1042/0264-6021:3540605. Biochem J. 2001. PMID: 11237865 Free PMC article. - Phosphorylation and nuclear exclusion of the forkhead transcription factor FKHR after epidermal growth factor treatment in human breast cancer cells.
Jackson JG, Kreisberg JI, Koterba AP, Yee D, Brattain MG. Jackson JG, et al. Oncogene. 2000 Sep 21;19(40):4574-81. doi: 10.1038/sj.onc.1203825. Oncogene. 2000. PMID: 11030146 - Similarities and differences between insulin and IGF-I: structures, receptors, and signalling pathways.
Werner H, Weinstein D, Bentov I. Werner H, et al. Arch Physiol Biochem. 2008 Feb;114(1):17-22. doi: 10.1080/13813450801900694. Arch Physiol Biochem. 2008. PMID: 18465355 Review. - Regulation of the FoxO family of transcription factors by phosphatidylinositol-3 kinase-activated signaling.
Arden KC, Biggs WH 3rd. Arden KC, et al. Arch Biochem Biophys. 2002 Jul 15;403(2):292-8. doi: 10.1016/s0003-9861(02)00207-2. Arch Biochem Biophys. 2002. PMID: 12139979 Review. No abstract available.
Cited by
- PPAR{alpha} mediates the hypolipidemic action of fibrates by antagonizing FoxO1.
Qu S, Su D, Altomonte J, Kamagate A, He J, Perdomo G, Tse T, Jiang Y, Dong HH. Qu S, et al. Am J Physiol Endocrinol Metab. 2007 Feb;292(2):E421-34. doi: 10.1152/ajpendo.00157.2006. Epub 2006 Sep 19. Am J Physiol Endocrinol Metab. 2007. PMID: 16985262 Free PMC article. - FoxO1 integrates insulin signaling to VLDL production.
Kamagate A, Dong HH. Kamagate A, et al. Cell Cycle. 2008 Oct;7(20):3162-70. doi: 10.4161/cc.7.20.6882. Epub 2008 Oct 27. Cell Cycle. 2008. PMID: 18927507 Free PMC article. Review. - Adipocyte HSL is required for maintaining circulating vitamin A and RBP4 levels during fasting.
Steinhoff JS, Wagner C, Dähnhardt HE, Košić K, Meng Y, Taschler U, Pajed L, Yang N, Wulff S, Kiefer MF, Petricek KM, Flores RE, Li C, Dittrich S, Sommerfeld M, Guillou H, Henze A, Raila J, Wowro SJ, Schoiswohl G, Lass A, Schupp M. Steinhoff JS, et al. EMBO Rep. 2024 Jul;25(7):2878-2895. doi: 10.1038/s44319-024-00158-x. Epub 2024 May 20. EMBO Rep. 2024. PMID: 38769419 Free PMC article. - The involvement of FoxO in cell survival and chemosensitivity mediated by Mirk/Dyrk1B in ovarian cancer.
Gao J, Yang X, Yin P, Hu W, Liao H, Miao Z, Pan C, Li N. Gao J, et al. Int J Oncol. 2012 Apr;40(4):1203-9. doi: 10.3892/ijo.2011.1293. Epub 2011 Dec 12. Int J Oncol. 2012. PMID: 22159921 Free PMC article. - Zerumbone suppresses IKKα, Akt, and FOXO1 activation, resulting in apoptosis of GBM 8401 cells.
Weng HY, Hsu MJ, Wang CC, Chen BC, Hong CY, Chen MC, Chiu WT, Lin CH. Weng HY, et al. J Biomed Sci. 2012 Oct 5;19(1):86. doi: 10.1186/1423-0127-19-86. J Biomed Sci. 2012. PMID: 23035900 Free PMC article.
References
- Abraham R.T. (1996) Immunopharmacology of rapamycin. Annu. Rev. Immunol., 14, 483–510. - PubMed
- Alessi D.R., Caudwell, F.B., Andjelkovic, M., Hemmings, B.A. and Cohen, P. (1996) Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett., 399, 333–338. - PubMed
- Alessi D.R. et al. (1997) 3-phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Biol., 7, 776–789. - PubMed
- Alessi D.R., Kozlowski, M.T., Weng, Q.P., Morrice, N. and Avruch, J. (1998) 3-phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr. Biol., 8, 69–81. - PubMed
- Anderson M.J., Viars, C.S., Czekay, S., Cavenee, W.K. and Arden, K.C. (1998) Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics, 47, 187–199. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous