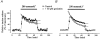Tyrosine phosphorylation modulates the osmosensitivity of volume-dependent taurine efflux from glial cells in the rat supraoptic nucleus - PubMed (original) (raw)
Tyrosine phosphorylation modulates the osmosensitivity of volume-dependent taurine efflux from glial cells in the rat supraoptic nucleus
C Deleuze et al. J Physiol. 2000.
Abstract
1. In the supraoptic nucleus, taurine, selectively released in an osmodependent manner by glial cells through volume-sensitive anion channels, is likely to inhibit neuronal activity as part of the osmoregulation of vasopressin release. We investigated the involvement of various kinases in the activation of taurine efflux by measuring [3H]taurine release from rat acutely isolated supraoptic nuclei. 2. The protein tyrosine kinase inhibitors genistein and tyrphostin B44 specifically reduced, but did not suppress, both the basal release of taurine and that evoked by a hypotonic stimulus. Inhibition of tyrosine phosphatase by orthovanadate had the opposite effect. 3. The tyrosine kinase and phosphatase inhibitors shifted the relationship between taurine release and medium osmolarity in opposite directions, suggesting that tyrosine phosphorylation modulates the osmosensitivity of taurine release, but is not necessary for its activation. 4. Genistein also increased the amplitude of the decay of the release observed during prolonged hypotonic stimulation. Potentiation of taurine release by tyrosine kinases could serve to maintain a high level of taurine release in spite of cell volume regulation. 5. Taurine release was unaffected by inhibitors and/or activators of PKA, PKC, MEK and Rho kinase. 6. Our results demonstrate a unique regulation by protein tyrosine kinase of the osmosensitivity of taurine efflux in supraoptic astrocytes. This points to the presence of specific volume-dependent anion channels in these cells, or to a specific activation mechanism or regulatory properties. This may relate to the particular role of the osmodependent release of taurine in this structure in the osmoregulation of neuronal activity.
Figures
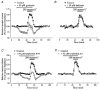
Figure 1. Inhibition of protein tyrosine kinase reduces taurine release
Taurine release from isolated SON perfused with control (300 mosmol l−1) and hyposmotic (280 mosmol l−1, grey bar) solutions. Release is expressed as percentage of control baseline. A, application of the PTK inhibitor genistein (50 μM), for the duration indicated by the open bar, decreases taurine release in both osmotic conditions (_n_= 5). B, the genistein inactive analogue daidzein has no effect (_n_= 3). C, the other PTK inhibitor tyrphostin B44 (50 μM) similarly reduces the osmodependent release of taurine (_n_= 4). D, tyrphostin A1, the tyrphostin inactive analogue, does not affect release (_n_= 4).
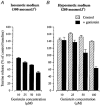
Figure 2. Dose dependency of genistein inhibitory effect
Inhibition of taurine release in isosmotic (A) and hyposmotic media (measured at the peak of the response to a stimulus of 280 mosmol l−1; B) by the different concentrations of genistein indicated. Data are expressed as percentage of control baseline. Number of observations is 3–5, except for inhibition of basal release by 50 μM genistein for which _n_= 22.
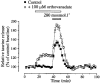
Figure 3. Orthovanadate potentiates taurine efflux
Inhibition of tyrosine phosphatase by 100 μM orthovanadate potentiates both basal taurine release in isosmotic medium and that during a hypotonic stimulus of 280 mosmol l−1 (_n_= 4).
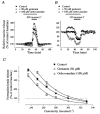
Figure 4. The level of tyrosine phosphorylation determines the osmosensitivity of taurine release
A and B, effects of genistein and orthovanadate on taurine release during osmotic stimulation with a medium of 250 mosmol l−1 (A) and 330 mosmol l−1 (B). Note the different scales on the _Y-_axis in A and B. C, relationships between the peak amplitude of taurine release and medium osmolarity in the absence (▪) and presence of genistein (^) or orthovanadate (▵). Peak release is expressed as percentage of control baseline measured at 300 mosmol l−1 in the absence of the drug. Relationships were modelled with sigmoid curves (Boltzmann equation, continuous lines). Genistein and orthovanadate shift the relationship to the left and to the right, respectively. Number of observations is 3 or 4 for orthovanadate (except at 300 mosmol l−1 where it is 14), 4 or 5 for genistein (except at 300 mosmol l−1 where it is 22), and 4–10 for the control.
Figure 5. Genistein modifies the decay kinetics of taurine release
Release of taurine induced by long duration (46 min) stimuli with hyposmotic solutions of 280 (A) and 250 mosmol l−1 (B) in the absence or continuous presence of 50 μM genistein. The responses display a decay component during the hypotonic stimulus. For comparison purposes, basal release is subtracted from the data and evoked release is normalised to the peak amplitude. The decay component is fitted with a monoexponential function (continuous lines). Genistein specifically increases the relative amplitude of this decay component of taurine release with no change in its time constant.
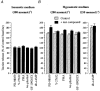
Figure 6. Other kinases are not involved in the regulation of taurine release
Bar graphs summarising the effects of activators and inhibitors of various other protein kinases on release of taurine in isosmotic conditions (A) and at the peak of the response to a hyposmotic stimulus (B). All compounds were tested with a stimulus of 280 mosmol l−1, except db-cAMP, which was tested with a stimulus of 250 mosmol l−1. Data (mean of 4–6 observations) are expressed as percentage of the control baseline. Compounds include inhibitors of MEK (PD-98059, 50 μM), and Rho kinase (Y-27632, 25 μM), an activator of PKC (PMA, 100 nM) and its inactive analogue 4α-phorbol (100 nM), an inhibitor of PKC (GF-109203X, 500 nM), and an activator of PKA (db-cAMP, 1 mM).
Similar articles
- Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus.
Deleuze C, Duvoid A, Hussy N. Deleuze C, et al. J Physiol. 1998 Mar 1;507 ( Pt 2)(Pt 2):463-71. doi: 10.1111/j.1469-7793.1998.463bt.x. J Physiol. 1998. PMID: 9518705 Free PMC article. - Pharmacological characterization of volume-sensitive, taurine permeable anion channels in rat supraoptic glial cells.
Brès V, Hurbin A, Duvoid A, Orcel H, Moos FC, Rabié A, Hussy N. Brès V, et al. Br J Pharmacol. 2000 Aug;130(8):1976-82. doi: 10.1038/sj.bjp.0703492. Br J Pharmacol. 2000. PMID: 10952690 Free PMC article. - Osmoregulation of vasopressin secretion via activation of neurohypophysial nerve terminals glycine receptors by glial taurine.
Hussy N, Brès V, Rochette M, Duvoid A, Alonso G, Dayanithi G, Moos FC. Hussy N, et al. J Neurosci. 2001 Sep 15;21(18):7110-6. doi: 10.1523/JNEUROSCI.21-18-07110.2001. J Neurosci. 2001. PMID: 11549721 Free PMC article. - Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure.
Hussy N, Deleuze C, Desarménien MG, Moos FC. Hussy N, et al. Prog Neurobiol. 2000 Oct;62(2):113-34. doi: 10.1016/s0301-0082(99)00071-4. Prog Neurobiol. 2000. PMID: 10828380 Review. - New role of taurine as an osmomediator between glial cells and neurons in the rat supraoptic nucleus.
Hussy N, Deleuze C, Brès V, Moos FC. Hussy N, et al. Adv Exp Med Biol. 2000;483:227-37. doi: 10.1007/0-306-46838-7_25. Adv Exp Med Biol. 2000. PMID: 11787602 Review. No abstract available.
Cited by
- Signaling events during swelling and regulatory volume decrease.
Pasantes-Morales H, Cardin V, Tuz K. Pasantes-Morales H, et al. Neurochem Res. 2000 Oct;25(9-10):1301-14. doi: 10.1023/a:1007652330703. Neurochem Res. 2000. PMID: 11059803 Review. - Regulation of the cellular content of the organic osmolyte taurine in mammalian cells.
Lambert IH. Lambert IH. Neurochem Res. 2004 Jan;29(1):27-63. doi: 10.1023/b:nere.0000010433.08577.96. Neurochem Res. 2004. PMID: 14992263 Review. - Influence of protein tyrosine kinases on cell volume change-induced taurine release.
Pasantes-Morales H, Franco R. Pasantes-Morales H, et al. Cerebellum. 2002 Apr;1(2):103-9. doi: 10.1080/147342202753671231. Cerebellum. 2002. PMID: 12882359 Review. - Insulin-like growth factor-1 inhibits adult supraoptic neurons via complementary modulation of mechanoreceptors and glycine receptors.
Ster J, Colomer C, Monzo C, Duvoid-Guillou A, Moos F, Alonso G, Hussy N. Ster J, et al. J Neurosci. 2005 Mar 2;25(9):2267-76. doi: 10.1523/JNEUROSCI.4053-04.2005. J Neurosci. 2005. PMID: 15745952 Free PMC article. - Astroglial Regulation of Magnocellular Neuroendocrine Cell Activities in the Supraoptic Nucleus.
Wang SC, Parpura V, Wang YF. Wang SC, et al. Neurochem Res. 2021 Oct;46(10):2586-2600. doi: 10.1007/s11064-020-03172-2. Epub 2020 Nov 20. Neurochem Res. 2021. PMID: 33216313 Free PMC article. Review.
References
- Basavappa S, Ellory JC. The role of swelling-induced anion channels during neuronal volume regulation. Molecular Neurobiology. 1996;13:137–153. - PubMed
- Boxall AR, Lancaster B. Tyrosine kinases and synaptic transmission. European Journal of Neuroscience. 1998;10:2–7. - PubMed
- Cannon CL, Basavappa S, Strange K. Intracellular ionic strength regulates the volume sensitivity of a swelling-activated anion channel. American Journal of Physiology. 1998;275:C416–422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources