P(0) glycoprotein overexpression causes congenital hypomyelination of peripheral nerves - PubMed (original) (raw)
. 2000 Mar 6;148(5):1021-34.
doi: 10.1083/jcb.148.5.1021.
M L Feltri, A Quattrini, D Imperiale, S Previtali, M D'Antonio, R Martini, X Yin, B D Trapp, L Zhou, S Y Chiu, A Messing
Affiliations
- PMID: 10704451
- PMCID: PMC2174542
- DOI: 10.1083/jcb.148.5.1021
P(0) glycoprotein overexpression causes congenital hypomyelination of peripheral nerves
L Wrabetz et al. J Cell Biol. 2000.
Abstract
We show that normal peripheral nerve myelination depends on strict dosage of the most abundantly expressed myelin gene, myelin protein zero (Mpz). Transgenic mice containing extra copies of Mpz manifested a dose-dependent, dysmyelinating neuropathy, ranging from transient perinatal hypomyelination to arrested myelination and impaired sorting of axons by Schwann cells. Myelination was restored by breeding the transgene into the Mpz-null background, demonstrating that dysmyelination does not result from a structural alteration or Schwann cell-extrinsic effect of the transgenic P(0) glycoprotein. Mpz mRNA overexpression ranged from 30-700%, whereas an increased level of P(0) protein was detected only in nerves of low copy-number animals. Breeding experiments placed the threshold for dysmyelination between 30 and 80% Mpz overexpression. These data reveal new points in nerve development at which Schwann cells are susceptible to increased gene dosage, and suggest a novel basis for hereditary neuropathy.
Figures
Figure 1
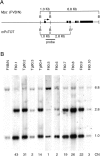
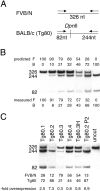
Southern blot analysis for transgene copy number. Southern blot analysis (B) revealed ten founders (F80), from which three lines were established (Tg80). A polymorphic BglII restriction site (B*) distinguishes the mP0TOT transgene from the endogenous Mpz FVB/N alleles (A). Copy numbers (CN) were estimated by the ratio of the 2.8–6.8-kb signal intensities.
Figure 2
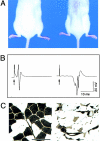
Phenotype of high copy number Tg80.2 animals. A, Tg80.2 animals at P120 manifested atrophy in axial and hindlimb muscles. B, Traces are shown of CMAP generated by sciatic nerve stimulation in P42 animals. Arrows indicate stimulation artifact. Note the markedly delayed onset and dispersion of the Tg80.2 CMAP, due to the slowed NCV. C, ATPase isotype staining (pH 4.6) in leg muscles revealed atrophy of many fibers and angulated fibers (arrows), suggesting denervation. Bar, 100 μm.
Figure 3
Semi-thin section analysis of sciatic nerve in Tg80.2. Transverse (A and C) or longitudinal (B and D) sections stained with toluidine blue from P28 Tg80.2 (C and D) as compared with wild-type (A and B) sciatic nerves showed a marked paucity of myelin (one internode of thin myelin is marked by a double asterisk) and an increased number of nuclei (arrowheads). In addition, bundles of unsorted axons surrounded by several Schwann cell nuclei were evident in every section (arrows; see also Fig. 4). No onion bulbs were seen. Bar, 20 μm.
Figure 4
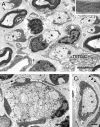
Ultrastructural analysis of Tg80.2 sciatic nerve showed dysmyelination. A, A typical transverse section of P28 sciatic nerve showed a mixture of occasional Schwann cells that had formed thin myelin sheaths of normal periodicity (inset), or more commonly, Schwann cells that had ensheathed single large axons, but had not advanced an inner mesaxon around them (asterisks). Nonmyelin-forming Schwann cells (nmsc) ensheathed small axons normally. Bar, 5 μm. B, Other Schwann cell families surrounded bundles of mixed caliber, naked axons; some of these Schwann cells were segregating large axons away from the bundles (arrow). Bar, 2 μm. C, Many Schwann cells were surrounded by pockets of redundant basal lamina, some containing collagen fibers (arrowheads). Bar, 1 μm.
Figure 5
Semi-thin section analysis of Tg80.2 sciatic nerves during postnatal development. Transverse sections stained with toluidine blue from wild-type (A, C, E, and G) or Tg80.2 (B, D, F, and H) animals at P5 (A and B), P15 (C and D), P45 (E and F), and P90 (G and H) demonstrate that dysmyelination was already present by P5 and remained throughout development. Arrows indicate bundles of unsorted axons. Extracellular myelin debris was not obvious at any age (see also Fig. 4). Bar, 50 μm.
Figure 6
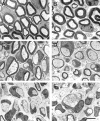
Dysmyelination parallels Mpz gene dosage. Low magnification electron micrographs of transverse sections of P28 sciatic nerve from FVB/N (A), Tg80.3 (B), Tg80.4 (C), Tg80.3 homozygote (D), Tg80.2 (E), and F80.8 (F) demonstrate that dysmyelination was absent in low copy number animals (B), moderate in middle copy number animals (C), and most pronounced in high copy number animals (E and F). Note that in contrast to Tg80.3 hemizygotes (B), Tg80.3 homozygotes manifested dysmyelination (D). Bar, 5 μm.
Figure 7
Semiquantitative RT-PCR analysis reveals Mpz overexpression. A, Total RNA prepared from sciatic nerve was reverse-transcribed; the product was amplified by PCR using a primer pair that recognized identically both endogenous (FVB/N) or Tg80 (BALB/c) P0 cDNAs. The FVB/N cDNA was distinguished by DpnII digestion, revealing 244- and 82-nucleotide (nt) products. B, Various mixtures of FVB/N (F) and BALB/c (B) reverse-transcribed product were analyzed to validate the method. The relative proportion (in percent of total signal) of FVB/N (326-nt band) or BALB/c (244 + 82-nt bands) were ± 5% of predicted in three separate experiments (one shown). C, Reverse-transcribed products from sciatic nerve total RNA from various transgenic nerves at P28 or P2 (Tg80.2 P2) were analyzed for the relative proportion of Tg80 P0 mRNA. Overexpression (-fold) was the quotient of Tg80 signal divided by endogenous signal. Note that the pairs, Tg80.4 and Tg80.3H (homozygote) or Tg80.2 P28 and Tg80.2 P2, manifested very similar ratios of overexpression. Undigested amplification product (uncut) revealed only the 326-nt band.
Figure 8
Dysmyelination is rescued in the Mpz null background. Tg80.4 was crossed into the Mpz heterozygous and homozygous null background and offspring were identified by Southern blotting (G). The Mpz null allele produced a 2.8-kb band due to the BglII polymorphism (see Fig. 1), and an additional 2.1-kb band due to the neomycin resistance gene. In the homozygous null animals, no 6.8-kb band appeared as the endogenous FVB/N Mpz alleles were absent. Tg80.4 was distinguished (both the Mpz null allele and Tg80.4 contain the BglII polymorphism) by the presence of a much more intense 1.0-kb band. Semi-thin section analysis of P28 sciatic nerves from 3–5 animals of each genotype showed that Tg80.4 homozygous null nerves (80.4/−/−; D) appeared normally myelinated as compared with nontransgenic (FVB/N; A) littermates, and as compared with the dysmyelinated Tg80.4 (B) or homozygous null (Mpz −/−; C) littermates. Note that at P28, heterozygous null (Mpz +/−; E) littermates were slightly hypomyelinated in the FVB/N congenic background, and that Tg80.4 heterozygous null nerves (80.4/+/−; F) showed a mixture of hypo- and hypermyelinated fibers. Bar, 20 μm.
Figure 9
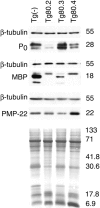
P0 overexpression is accompanied by alteration of other myelin protein levels. Western blot analysis was performed on P28 sciatic nerve lysates from Tg80 animals with dysmyelination ranging from none (Tg80.3), to moderate (Tg80.4), to severe (Tg80.2). Dysmyelination was associated with proportionally decreased total P0, and high molecular weight isoforms of MBP consistent with developmental delay. In contrast, Tg80.3 nerves showed ∼60% increased P0 and mature MBP isoforms. Note that MBP and PMP22 levels were reduced in Tg80.3 nerves, even when normalized to tubulin or amido black staining of the blot for total protein. Numbers × 1,000 indicate approximate M r.
Similar articles
- Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function.
Chen S, Velardez MO, Warot X, Yu ZX, Miller SJ, Cros D, Corfas G. Chen S, et al. J Neurosci. 2006 Mar 22;26(12):3079-86. doi: 10.1523/JNEUROSCI.3785-05.2006. J Neurosci. 2006. PMID: 16554459 Free PMC article. - Peripheral nerve dysmyelination due to P0 glycoprotein overexpression is dose-dependent.
Quattrini A, Feltri ML, Previtali S, Fasolini M, Messing A, Wrabetz L. Quattrini A, et al. Ann N Y Acad Sci. 1999 Sep 14;883:294-301. Ann N Y Acad Sci. 1999. PMID: 10586254 - A nonsense mutation in myelin protein zero causes congenital hypomyelination neuropathy through altered P0 membrane targeting and gain of abnormal function.
Fratta P, Ornaghi F, Dati G, Zambroni D, Saveri P, Belin S, D'Adamo P, Shy M, Quattrini A, Laura Feltri M, Wrabetz L. Fratta P, et al. Hum Mol Genet. 2019 Jan 1;28(1):124-132. doi: 10.1093/hmg/ddy336. Hum Mol Genet. 2019. PMID: 30239779 Free PMC article. - Tetraspan myelin protein PMP22 and demyelinating peripheral neuropathies: new facts and hypotheses.
Müller HW. Müller HW. Glia. 2000 Jan 15;29(2):182-5. doi: 10.1002/(sici)1098-1136(20000115)29:2<182::aid-glia12>3.3.co;2-b. Glia. 2000. PMID: 10625337 Review. - Role of immune cells in animal models for inherited peripheral neuropathies.
Wang Ip C, Kroner A, Fischer S, Berghoff M, Kobsar I, Mäurer M, Martini R. Wang Ip C, et al. Neuromolecular Med. 2006;8(1-2):175-90. doi: 10.1385/nmm:8:1-2:175. Neuromolecular Med. 2006. PMID: 16775375 Review.
Cited by
- Genetic and clinical spectrums in Korean Charcot-Marie-Tooth disease patients with myelin protein zero mutations.
Kim HJ, Nam SH, Kwon HM, Lim SO, Park JH, Kim HS, Kim SB, Lee KS, Lee JE, Choi BO, Chung KW. Kim HJ, et al. Mol Genet Genomic Med. 2021 Jun;9(6):e1678. doi: 10.1002/mgg3.1678. Epub 2021 Apr 6. Mol Genet Genomic Med. 2021. PMID: 33825325 Free PMC article. - Pathology of a mouse mutation in peripheral myelin protein P0 is characteristic of a severe and early onset form of human Charcot-Marie-Tooth type 1B disorder.
Rünker AE, Kobsar I, Fink T, Loers G, Tilling T, Putthoff P, Wessig C, Martini R, Schachner M. Rünker AE, et al. J Cell Biol. 2004 May 24;165(4):565-73. doi: 10.1083/jcb.200402087. Epub 2004 May 17. J Cell Biol. 2004. PMID: 15148307 Free PMC article. - The role of combined SNV and CNV burden in patients with distal symmetric polyneuropathy.
Pehlivan D, Beck CR, Okamoto Y, Harel T, Akdemir ZH, Jhangiani SN, Withers MA, Goksungur MT, Carvalho CM, Czesnik D, Gonzaga-Jauregui C, Wiszniewski W, Muzny DM, Gibbs RA, Rautenstrauss B, Sereda MW, Lupski JR. Pehlivan D, et al. Genet Med. 2016 May;18(5):443-51. doi: 10.1038/gim.2015.124. Epub 2015 Sep 17. Genet Med. 2016. PMID: 26378787 Free PMC article. - Resetting translational homeostasis restores myelination in Charcot-Marie-Tooth disease type 1B mice.
D'Antonio M, Musner N, Scapin C, Ungaro D, Del Carro U, Ron D, Feltri ML, Wrabetz L. D'Antonio M, et al. J Exp Med. 2013 Apr 8;210(4):821-38. doi: 10.1084/jem.20122005. Epub 2013 Apr 1. J Exp Med. 2013. PMID: 23547100 Free PMC article. - Loss of Fig4 in both Schwann cells and motor neurons contributes to CMT4J neuropathy.
Vaccari I, Carbone A, Previtali SC, Mironova YA, Alberizzi V, Noseda R, Rivellini C, Bianchi F, Del Carro U, D'Antonio M, Lenk GM, Wrabetz L, Giger RJ, Meisler MH, Bolino A. Vaccari I, et al. Hum Mol Genet. 2015 Jan 15;24(2):383-96. doi: 10.1093/hmg/ddu451. Epub 2014 Sep 3. Hum Mol Genet. 2015. PMID: 25187576 Free PMC article.
References
- Adlkofer K., Martini R., Aguzzi A., Zielasek J., Toyka K.V., Suter U. Hypermyelination and demyelinating peripheral neuropathy in Pmp22-deficient mice. Nat. Genet. 1995;11:274–280. - PubMed
- Archelos J.J., Roggenbuck K., Schneider-Schaulies J., Linington C., Toyka K.V., Hartung H.P. Production and characterization of monoclonal antibodies to the extracellular domain of P0 . J. Neurosci. Res. 1993;35:46–53. - PubMed
- Barbarese E., Carson J.H., Braun P. Accumulation of the four myelin basic proteins in mouse brain during development. J. Neurochem. 1978;31:779–782. - PubMed
- Bermingham J.R., Jr., Scherer S.S., O'Connell S., Arroyo E., Kalla K.A., Powell F.L., Rosenfeld M.G. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 1996;10:1751–1762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS-38186/NS/NINDS NIH HHS/United States
- R01 NS038186/NS/NINDS NIH HHS/United States
- R01 NS023375/NS/NINDS NIH HHS/United States
- R37 NS038186/NS/NINDS NIH HHS/United States
- NS-23375/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases