Application of neutralizing antibodies against NI-35/250 myelin-associated neurite growth inhibitory proteins to the adult rat cerebellum induces sprouting of uninjured purkinje cell axons - PubMed (original) (raw)
Application of neutralizing antibodies against NI-35/250 myelin-associated neurite growth inhibitory proteins to the adult rat cerebellum induces sprouting of uninjured purkinje cell axons
A Buffo et al. J Neurosci. 2000.
Abstract
The myelin-associated proteins NI-35/250 exert a powerful inhibition on axon regeneration, but their function exerted on intact neurons is still unclear. In the adult CNS these proteins are thought to regulate axon growth processes to confine plasticity within restricted regions and to prevent the formation of aberrant connections. We have recently shown that application of neutralizing IN-1 antibody Fab fragment against NI-35/250 proteins to the adult cerebellum induces the expression of injury/growth-associated markers in intact Purkinje cells. Here, we asked whether these cellular modifications are accompanied by growth phenomena of Purkinje neurites. A single intraparenchymal application of IN-1 Fab fragment to the adult cerebellum induces a profuse sprouting of Purkinje axons along their intracortical course. The newly formed processes spread to cover most of the granular layer depth. A significant axon outgrowth is evident 2 d after injection; it tends to increase at 5 and 7 d, but it is almost completely reversed after 1 month. No axonal modifications occur in control Fab-treated cerebella. The IN-1 Fab fragment-induced cellular changes and axon remodeling are essentially reproduced by applying affinity-purified antibody 472 raised against a peptide sequence of the recombinant protein NI-220, thus confirming the specificity of the applied treatments on these myelin-associated molecules. Functional neutralization of NI-35/250 proteins induces outgrowth from uninjured Purkinje neurites in the adult cerebellum. Together with previous observations, this suggests that these molecules regulate axonal plasticity to maintain the proper targeting of terminal arbors within specific gray matter regions.
Figures
Fig. 1.
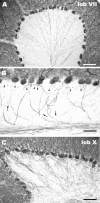
Morphology of Purkinje axons in the intact cerebellum. A shows the typical pattern of calbindin-immunostained Purkinje axons, originating from the basal perikaryal pole and converging toward the axial white matter of the folium. At a higher magnification (B), the thinner recurrent collateral branches (arrows), which ascend through the granular layer to end in the infraganglionic plexus (arrowheads), can be disclosed. This pattern is consistently observed over the whole cerebellar cortex except for lobules IX (ventral portion) and X (C). Here, the recurrent Purkinje axon branches form a thick terminal plexus covering the whole granule cell layer. Scale bars: A,C, 100 μm; B, 50 μm.
Fig. 2.
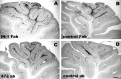
Structural features of the intracortical Purkinje axons in Fab fragment-treated cerebella. The diagram_shows a sagittal section of the cerebellar vermis; the dark gray area indicates the dorsal vermal lobules that were affected by the injections (asterisks indicate the approximate positions of the injection sites), whereas the light gray area defines the ventral vermal lobules, examined as an internal control (see Materials and Methods and Results). The_rectangles indicate the approximate position of the images shown in the relevant micrographs. A and_B_ display a portion of lobules VII and I, respectively, taken from the same cerebellar section of a rat treated with the IN-1 Fab fragment at 7 d survival time. In A, the granular layer is covered by a huge number of thin axon profiles running in all directions, among which the thicker and straight-running Purkinje stem axons can be disclosed. In contrast, only the stem axons can be seen in the granular layer in B, and the few terminal branches are restricted to the vicinity of Purkinje cell somata. C and D show the tip of lobule VIa from two different cerebella 5 d after IN-1 or control Fab injection, respectively. Note the numerous Purkinje axon branches in the granular layer in C, which are not present in the same area of the control Fab-treated cerebellum (D). Scale bars, 100 μm.
Fig. 3.
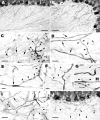
Morphology of Purkinje axon sprouts in IN-1 Fab fragment-treated cerebella. A and B show a control and an IN-1 Fab fragment-treated cerebellum, respectively. The newly formed sprouts (B) form a rather dense network of randomly oriented processes with some varicose branches, among which the Purkinje stem axons can be recognized because of their straight course and thicker caliber. The Purkinje axon in_C_ (small arrows) is characterized by a thickened segment from which several short sprouts (arrowheads), bearing one or a few varicosities, and another long slender process (large arrows) originate.Arrows in D point to Purkinje stem axons coursing through the granular layer; two thin branches (arrowheads) emerge at the same site along one of such axons and take divergent routes. A thin varicose chain (E, arrowheads) buds from a thickened segment of a Purkinje axon stem (arrow). Another Purkinje axon stem (F) emits three processes (arrowheads) along its way; the course of the longest of such branches suggests that it may be a recurrent collateral.Arrowheads in G and H_point to short stubby sprouts emitted by thick Purkinje axon stems in the deepest portions of the granular layer. The Purkinje axon in I (arrow) gives rise to a long branch (arrowheads) bearing several varicosities localized in its distalmost segment. J shows an area from the dorsal cerebellum 30 d after IN-1 Fab fragment injection. Most of the sprouts in the granular layer have disappeared, although some scattered processes with a random course (arrowheads) still persist. Survival times were 2 d in C,G, and H, 5 d in I, 7 d in A, B, and_D_–_F, and 30 d in J. Scale bars: A, B, 50 μm;J, 30 μm; C, D,I, 10 μm; E_–_H, 5 μm.
Fig. 4.
Activation of microglia by Fab fragment or antibody injections to the cerebellum. The micrographs show microglia stained by G. simplicifolia isolectin B4 in cerebellar sections 2 d after injections of IN-1 Fab fragment (A), control Fab fragment (B), antibody 472 (C), and control antibody (D). All of the injections induced a similar recruitment of ameboid elements in the vicinity of injection sites and increased density of branched cells in the adjacent cortex. Scale bar, 500 μm.
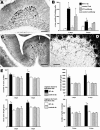
Fig. 5.
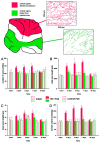
Quantitative analysis of IN-1 Fab fragment-induced Purkinje axon sprouting. The _diagram_illustrates a sagittal section of the vermis; the _red_labeling indicates the dorsal vermal lobules affected by the injections (asterisks indicate the approximate positions of the injections sites), whereas the _green_labeling defines the ventral vermal lobules, examined as an internal control (see Results). In addition, two representative 200 × 250 μm areas taken from dorsal and ventral cortex, respectively, are displayed in which all Purkinje axon profiles have been reproduced and measured. A_–_D show the results of the morphometric analysis performed on such sampled areas (for details, see Materials and Methods and Results). No differences exist between the values obtained from dorsal (red labeling of columns) and ventral (green labeling of columns) lobules in intact (cross-hatched columns) and control Fab-treated animals (hatched columns). In contrast, after injection of IN-1 Fab fragment (solid columns), all of the values from the dorsal lobules are consistently higher than those from the ventral lobules at 2, 5, and 7 d, but they return to control levels at 30 d. Asterisks indicate values that are significantly different from the relevant internal control (for details, see Results).
Fig. 6.
A_–_D, Effects of antiserum 472 application on adult intact Purkinje cells.A shows an anti-c-Jun-labeled section from the dorsal cerebellum 2 d after antibody injection; _arrowheads_point to several immunoreactive Purkinje cell nuclei. Quantification of c-Jun-immunolabeled Purkinje cells (B) reveals that the effect of antiserum 472 treatment is milder than that observed in IN-1 Fab fragment-treated rats (Zagrebelsky et al., 1998) but highly different from control antibody-treated rats. Five days after application of the 472 antibody, several Purkinje cells (C, arrowheads) throughout the dorsal vermal cortex become strongly reactive for NADPH diaphorase histochemistry. The sprouting of Purkinje axons is evident in anti-calbindin-immunostained sections (D, at 2 d survival time). Quantitative analysis of Purkinje axons (E) reveals a consistent increase of all the parameters calculated from dorsal vermal lobules (solid columns) of antibody 472-treated rats compared with those from ventral lobules (open columns). In contrast, no differences exist between dorsal (cross-hatched columns) and ventral (hatched columns) lobules from the cerebella receiving control antibody injections.Asterisks in B and _E_indicate values that are significantly different from the relevant internal control. Scale bars: A, 100 μm;C, 200 μm; D, 50 μm.
Similar articles
- Degradation of chondroitin sulfate proteoglycans induces sprouting of intact purkinje axons in the cerebellum of the adult rat.
Corvetti L, Rossi F. Corvetti L, et al. J Neurosci. 2005 Aug 3;25(31):7150-8. doi: 10.1523/JNEUROSCI.0683-05.2005. J Neurosci. 2005. PMID: 16079397 Free PMC article. - Cell-autonomous mechanisms and myelin-associated factors contribute to the development of Purkinje axon intracortical plexus in the rat cerebellum.
Gianola S, Savio T, Schwab ME, Rossi F. Gianola S, et al. J Neurosci. 2003 Jun 1;23(11):4613-24. doi: 10.1523/JNEUROSCI.23-11-04613.2003. J Neurosci. 2003. PMID: 12805301 Free PMC article. - Retrograde regulation of growth-associated gene expression in adult rat Purkinje cells by myelin-associated neurite growth inhibitory proteins.
Zagrebelsky M, Buffo A, Skerra A, Schwab ME, Strata P, Rossi F. Zagrebelsky M, et al. J Neurosci. 1998 Oct 1;18(19):7912-29. doi: 10.1523/JNEUROSCI.18-19-07912.1998. J Neurosci. 1998. PMID: 9742159 Free PMC article. - Regulation of intrinsic regenerative properties and axonal plasticity in cerebellar Purkinje cells.
Rossi F, Buffo A, Strata P. Rossi F, et al. Restor Neurol Neurosci. 2001;19(1-2):85-94. Restor Neurol Neurosci. 2001. PMID: 12085795 Review. - Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system.
Buchli AD, Schwab ME. Buchli AD, et al. Ann Med. 2005;37(8):556-67. doi: 10.1080/07853890500407520. Ann Med. 2005. PMID: 16338758 Review.
Cited by
- The strange case of Purkinje axon regeneration and plasticity.
Rossi F, Gianola S, Corvetti L. Rossi F, et al. Cerebellum. 2006;5(2):174-82. doi: 10.1080/14734220600786444. Cerebellum. 2006. PMID: 16818392 Review. - Degradation of chondroitin sulfate proteoglycans induces sprouting of intact purkinje axons in the cerebellum of the adult rat.
Corvetti L, Rossi F. Corvetti L, et al. J Neurosci. 2005 Aug 3;25(31):7150-8. doi: 10.1523/JNEUROSCI.0683-05.2005. J Neurosci. 2005. PMID: 16079397 Free PMC article. - NogoA restricts synaptic plasticity in the adult hippocampus on a fast time scale.
Delekate A, Zagrebelsky M, Kramer S, Schwab ME, Korte M. Delekate A, et al. Proc Natl Acad Sci U S A. 2011 Feb 8;108(6):2569-74. doi: 10.1073/pnas.1013322108. Epub 2011 Jan 24. Proc Natl Acad Sci U S A. 2011. PMID: 21262805 Free PMC article. - Functional reorganization of the motor cortex in adult rats after cortical lesion and treatment with monoclonal antibody IN-1.
Emerick AJ, Neafsey EJ, Schwab ME, Kartje GL. Emerick AJ, et al. J Neurosci. 2003 Jun 15;23(12):4826-30. doi: 10.1523/JNEUROSCI.23-12-04826.2003. J Neurosci. 2003. PMID: 12832504 Free PMC article. - Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A.
Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K. Merkler D, et al. J Neurosci. 2001 May 15;21(10):3665-73. doi: 10.1523/JNEUROSCI.21-10-03665.2001. J Neurosci. 2001. PMID: 11331396 Free PMC article.
References
- Bähr M, Schwab ME. Antibody that neutralizes myelin-associated inhibitors of axon growth does not interfere with recognition of target-specific guidance information by retinal axons. J Neurobiol. 1996;30:281–292. - PubMed
- Bandtlow C, Schiweck W, Tai HH, Schwab ME, Skerra A. The Escherichia coli-derived Fab fragment of the IgM/kappa antibody IN-1 recognizes and neutralizes myelin-associated inhibitors of neurite growth. Eur J Biochem. 1996;241:468–475. - PubMed
- Bishop GA. The pattern of distribution of the local axonal collaterals of Purkinje cells in the intermediate cortex of the anterior lobe and paramedian lobule of the cat cerebellum. J Comp Neurol. 1982;210:1–9. - PubMed
- Blank NK, Seil FJ, Herndon RM. An ultrastructural study of cortical remodelling in cytosine arabinoside induced granuloprival cerebellum in tissue culture. Neuroscience. 1982;7:1509–1531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources