Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells - PubMed (original) (raw)
Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells
F F Bukauskas et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Communication-incompetent cell lines were transfected with connexin (Cx) 43 fused with enhanced green fluorescent protein (EGFP) to examine the relation between Cx distribution determined by fluorescence microscopy and electrical coupling measured at single-channel resolution in living cell pairs. Cx43-EGFP channel properties were like those of wild-type Cx43 except for reduced sensitivity to transjunctional voltage. Cx43-EGFP clustered into plaques at locations of cell-cell contact. Coupling was always absent in the absence of plaques and even in the presence of small plaques. Plaques exceeding several hundred channels always conferred coupling, but only a small fraction of channels were functional. These data indicate that clustering may be a requirement for opening of gap junction channels.
Figures
Figure 1
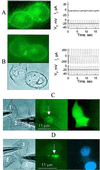
Cx43–EGFP fluorescence forms junctional plaques correlated with electrical coupling and dye transfer. (A) N2A cell pair showing only diffuse Cx43–EGFP fluorescence staining in appositional and other membrane areas (Left) was not electrically coupled (Right). Voltage steps applied to one cell (transjunctional voltage, _V_j,Lower trace) produced no changes in junctional current (_I_j) recorded in the unstepped cell. (B) A cell pair showing two junctional plaques and diffuse membrane staining elsewhere (Left, fluorescent and phase-contrast images) was electrically coupled as indicated by the large changes in _I_j in response to an applied _V_j. Coupling conductance was ≈12 nS. (C and D) Electrically coupled HeLa cells containing Cx43–EGFP plaques show Lucifer Yellow and DAPI cell–cell transfer. (Left) Phase. (Center) Fluorescence before dye injection. (Right) After dye injection. (C) A cluster of three cells. Lucifer Yellow was injected into cell 2 and transferred to cell 1 but not cell 3. A large (arrow) and a small plaque could be seen between cells 1 and 2;_g_j = 15 nS. No plaques were evident between cells 2 and 3. The fluorescent image (taken 5 min after dye loading of cell 2) showed spread to cell 1 but not cell 2. (D) A cluster of three cells. DAPI was injected into cell 2 and transferred to cell 1 but not cell 3. Four junctional plaques could be seen between cell 1 and cell 3;_g_j = 40 nS; however, no plaques were seen with cell 2. The fluorescent image taken 14 min after dye loading of cell 3 showed labeling of the nuclei of cells 1 and 3 but not cell 2.
Figure 2
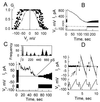
Voltage dependence of Cx43–EGFP GJs. (A) Junctional conductance depended on _V_j. The solid line is a fit of the data to a Boltzmann equation of the form_G_j = (1 −_G_min)/{1 + exp[A*(_V_j −_V_0)]} + _G_min, where _G_j is _g_j normalized to the maximum value, _g_max, at_V_j = 0; _G_min is the normalized residual _g_j;A is a measure of voltage sensitivity in mV−1; and _V_0 is the_V_j at which _G_j is halfway between the maximum and minimum values. The Boltzmann parameters were _V_0 = 81 ± 4 mV and A = 0.08 ± 0.02 mV−1, for positive V_j, and_V_0 = −82 ± 4 mV and_A = 0.09 ± 0.02 mV−1, for negative _V_j. _G_min was close to zero for both polarities of _V_j. (B) An example of the slow time course with which_I_j nears zero in response to a long-duration (2.5-min) _V_j of −90 mV (HeLa cell pair). Repeated −20-mV _V_j pulses, applied at 1-sec intervals before and after the long-duration_V_j step, show a slow_g_j recovery. (C)_V_j-induced gating of Cx43–EGFP GJs at the single-channel level in a HeLa cell pair that was coupled by nine functional channels. A _V_j of −100 mV was applied for a duration of 60 sec. Seven transitions ≈110 pS in size were evident (see Inset histogram of_I_j amplitude). There was no residual_g_j at the end of the_V_j step. (D) Linearity of the_I_j–_V_j relationship at the single-channel level. _V_j ramps of ±100 mV and 2-sec duration were applied at regular intervals preceded by brief (200-msec) ±40-mV _V_j steps. The arrow indicates spontaneous opening of a single Cx43–EGFP channel between a HeLa cell pair.
Figure 3
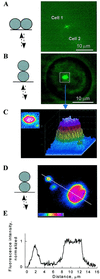
Fluorescence intensity reaches a plateau in the center of_en face_ images of larger Cx43–EGFP plaques. Schematic drawings (Left) show orientations of the cell pairs and light paths for excitation (upward arrows) and emission (downward arrows). (A and B) Side-by-side and_en face_ views of a Cx43–EGFP plaque in an N2A cell pair. (C) Two- and three-dimensional pseudocolor plots of fluorescence intensity in the square shown in B show a plateau in the central region of the plaque. (D)En face views of large and small plaques in a HeLa cell pair. (E) Plot of fluorescence intensity along the line shown in D crossing two of the three plaques. Fluorescence intensity is normalized to the average plateau value in the center of the largest plaque. Peak fluorescence was smaller in small plaques, but fall-off at edges was about the same. Pseudocolor in_C_ and D was adjusted to maximize the range of fluorescence intensity.
Figure 4
Relation between the size of Cx43–EGFP plaques and_g_j. Data are summarized from N2A, HeLa, and RIN cell pairs with single plaques. (A) Side-by-side view of an N2A cell pair containing a single junctional plaque ≈0.3 μm in diameter. _g_j in this cell pair was 230 pS, which corresponds to two functional channels. (B) Examination of the effect of focus (_z_-axis position) and size of the region of interest (ROI) used to measure fluorescence on the total measured fluorescence (_L_tot). Diagrams of a cell pair containing a single plaque (thick vertical bar) illustrate the experimental procedures used. The Upper drawing is a side view, in which the solid horizontal line represents the coverslip and the dashed horizontal lines represent different _z_-axis positions (z = 0 is the position giving the sharpest image of the plaque). The Lower drawing is the view as seen through the microscope. The concentric circles represent ROIs of different diameters, d. L_tot as a function of z is plotted for ROIs of varying diameters (indicated to the right of each plot). The cell pair from which the data were obtained had a single plaque ≈1 μm in diameter. The objective was moved upward in 0.5-μm steps starting at_z = 0. (C) Relation between the number of channels in a plaque and _g_j. Data were obtained from the three cell types: HeLa (circles), N2A (triangles), and RIN (squares). (Inset) An expanded view of data with _g_j between 0 and 0.8 nS is shown. _g_j was measured at small_V_js (<50 mV). (D) Plot of the calculated fraction of functional channels in a plaque vs.g_j from the data presented in_C. The solid line is a linear fit of the data for_g_js exceeding 4 nS. The dashed lines represent 99% confidence intervals.
Similar articles
- Gating properties of gap junction channels assembled from connexin43 and connexin43 fused with green fluorescent protein.
Bukauskas FF, Bukauskiene A, Bennett MV, Verselis VK. Bukauskas FF, et al. Biophys J. 2001 Jul;81(1):137-52. doi: 10.1016/S0006-3495(01)75687-1. Biophys J. 2001. PMID: 11423402 Free PMC article. - Coupling asymmetry of heterotypic connexin 45/ connexin 43-EGFP gap junctions: properties of fast and slow gating mechanisms.
Bukauskas FF, Angele AB, Verselis VK, Bennett MV. Bukauskas FF, et al. Proc Natl Acad Sci U S A. 2002 May 14;99(10):7113-8. doi: 10.1073/pnas.032062099. Proc Natl Acad Sci U S A. 2002. PMID: 12011467 Free PMC article. - Dominant-negative connexin43-EGFP inhibits calcium-transient synchronization of primary neonatal rat cardiomyocytes.
Oyamada Y, Zhou W, Oyamada H, Takamatsu T, Oyamada M. Oyamada Y, et al. Exp Cell Res. 2002 Feb 1;273(1):85-94. doi: 10.1006/excr.2001.5411. Exp Cell Res. 2002. PMID: 11795949 - Connexin-GFPs shed light on regulation of cell-cell communication by gap junctions.
Verselis VK, Bukauskas FF. Verselis VK, et al. Curr Drug Targets. 2002 Dec;3(6):483-99. doi: 10.2174/1389450023347272. Curr Drug Targets. 2002. PMID: 12448699 Review. - High resolution, fluorescence deconvolution microscopy and tagging with the autofluorescent tracers CFP, GFP, and YFP to study the structural composition of gap junctions in living cells.
Falk MM, Lauf U. Falk MM, et al. Microsc Res Tech. 2001 Feb 1;52(3):251-62. doi: 10.1002/1097-0029(20010201)52:3<251::AID-JEMT1011>3.0.CO;2-#. Microsc Res Tech. 2001. PMID: 11180618 Review.
Cited by
- Inhibition of histone deacetylase (HDAC) by 4-phenylbutyrate results in increased junctional conductance between rat corpora smooth muscle cells.
Wang HZ, Rosati B, Gordon C, Valiunas V, McKinnon D, Cohen IS, Brink PR. Wang HZ, et al. Front Pharmacol. 2015 Feb 3;6:9. doi: 10.3389/fphar.2015.00009. eCollection 2015. Front Pharmacol. 2015. PMID: 25691868 Free PMC article. - Polyamines regulate gap junction communication in connexin 43-expressing cells.
Shore L, McLean P, Gilmour SK, Hodgins MB, Finbow ME. Shore L, et al. Biochem J. 2001 Jul 15;357(Pt 2):489-95. doi: 10.1042/0264-6021:3570489. Biochem J. 2001. PMID: 11439099 Free PMC article. - Effect of angiotensin II and ethanol on the expression of connexin 43 in WB rat liver epithelial cells.
Bokkala S, Reis HM, Rubin E, Joseph SK. Bokkala S, et al. Biochem J. 2001 Aug 1;357(Pt 3):769-77. doi: 10.1042/0264-6021:3570769. Biochem J. 2001. PMID: 11463347 Free PMC article. - Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion.
Hunter AW, Barker RJ, Zhu C, Gourdie RG. Hunter AW, et al. Mol Biol Cell. 2005 Dec;16(12):5686-98. doi: 10.1091/mbc.e05-08-0737. Epub 2005 Sep 29. Mol Biol Cell. 2005. PMID: 16195341 Free PMC article. - Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis?
Griffith TM. Griffith TM. Br J Pharmacol. 2004 Mar;141(6):881-903. doi: 10.1038/sj.bjp.0705698. Br J Pharmacol. 2004. PMID: 15028638 Free PMC article. Review.
References
- Willecke K, Hennemann H, Dahl E, Jungbluth S, Heynkes R. Eur J Cell Biol. 1991;56:1–7. - PubMed
- Goodenough D A, Goliger J A, Paul D L. Annu Rev Biochem. 1996;65:475–502. - PubMed
- Bergoffen J, Scherer S S, Wang S, Scott M O, Bone L J, Paul D L, Chen K, Lensch M W, Chance P F, Fischbeck K H. Science. 1993;262:2039–2042. - PubMed
- Ionasescu V, Searby C, Ionasescu R. Hum Mol Genet. 1994;3:355–358. - PubMed
- Kelsell D P, Dunlop J, Stevens H P, Lench N J, Liang J N, Parry G, Mueller R F, Leigh I M. Nature (London) 1997;387:80–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM54179/GM/NIGMS NIH HHS/United States
- NS367060/NS/NINDS NIH HHS/United States
- R01 GM055632/GM/NIGMS NIH HHS/United States
- R01 NS036706/NS/NINDS NIH HHS/United States
- R01 GM054179/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous