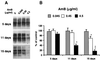Amphotericin B inhibits the generation of the scrapie isoform of the prion protein in infected cultures - PubMed (original) (raw)
Amphotericin B inhibits the generation of the scrapie isoform of the prion protein in infected cultures
A Mangé et al. J Virol. 2000 Apr.
Abstract
Transmissible spongiform encephalopathies form a group of fatal neurodegenerative disorders that have the unique property of being infectious, sporadic, or genetic in origin. Although some doubts about the nature of the responsible agent of these diseases remain, it is clear that a protein called PrP(Sc) plays a central role. PrP(Sc) is a conformational variant of PrP(C), the normal host protein. Polyene antibiotics such as amphotericin B have been shown to delay the accumulation of PrP(Sc) and to increase the incubation time of the disease after experimental transmission in laboratory animals. Unlike for Congo red and sulfated polyanions, no effect of amphotericin B has been observed in infected cultures. We show here for the first time that amphotericin B can inhibit PrP(Sc) generation in scrapie-infected GT1-7 and N2a cells. Its activity seems to be related to a modification of the properties of detergent-resistant microdomains. These results provide new insights into the mechanism of action of amphotericin B and confirm the usefulness of infected cultures in the therapeutic research of transmissible spongiform encephalopathies.
Figures
FIG. 1
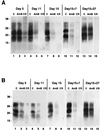
AmB inhibits PrPSc production in infected GT1-7 and S12 cell lines. Infected GT1-7 (A) and S12 (B) cells were passaged for the indicated periods of time in the absence (control [C]) or the presence of AmB (4.5 μg/ml) or CR (1 μg/ml) for up to 15 days (lanes 1 to 9). The drugs were then removed and cells were passaged in their absence for 7 and 27 additional days (lanes 10 to 15). PrPSc at each time point was detected by Western blotting after limited PK digestion as indicated in Materials and Methods. PrPSc signal disappeared rapidly upon CR treatment in both cell lines. AmB also reduced the amount of PrPSc detected, but at a lower rate; its signal did not disappear completely and was restored after removal of the drug. Molecular masses, on the left, are in kilodaltons.
FIG. 2
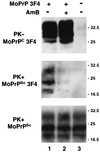
AmB inhibits the generation of PrPSc molecules after transfection of infected cells. Infected S12 cells were left untransfected (lane 3) or were transfected with 3F4-tagged MoPrP (lanes 1 and 2). When indicated, AmB (4.5 μg/ml) was added to the culture medium from the time of transfection (lane 2). Seventy-two hours after transfection, cells were lysed, and transfected 3F4-tagged MoPrPC was detected by Western blotting using Pri308 (upper panel). Lysates were subjected to limited PK digestion, and 3F4-tagged and total MoPrPSc was detected using Pri308 (middle panel) or a mixture of MAbs SAF 60, 69, and 70 (lower panel), respectively. Molecular masses, on the right, are in kilodaltons.
FIG. 3
Dose response and time course of action of AmB. (A) Infected S12 cells were cultured for 5, 11, and 15 days in the presence of various concentrations of AmB. At each time point, PrPSc was detected by immunoblotting after protease digestion as described in Materials and Methods. A decrease in PrPSc signal was observed at concentrations of 0.45 μg/ml and higher. (B) MoPrP-specific bands from panel A and from two other experiments were quantitated by densitometry. The amount of MoPrP remaining after digestion at each time point was plotted as a percentage of the control without AmB. Each bar represents the mean + the standard deviation. Values that are significantly different from those of untreated samples by paired t test are indicated by single (P < 0.05) and double (P < 0.005) asterisks.
FIG. 4
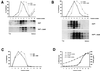
AmB affects flotation of PrPC and PrPSc. GT1-7 cells (A) or scrapie-infected GT1-7 cells (B) were incubated for 3 days with or without AmB (4.5 μg/ml). Cells were then collected and flotation gradients were performed as indicated in Materials and Methods. (A and B) Gradient fractions were methanol precipitated (A) or digested with PK (20 μg/ml, 30 min at 37°C) and ultracentrifuged (B). Pellets were resuspended in SDS loading buffer, and MoPrP-specific bands were detected by Western blotting using antibody P45-66 (A) or a mixture of MAbs SAF 60, 69, and 70 (B). In panel A, only 1/10 of fraction 7 and 1/30 of fractions 8 to 10 were loaded. Quantitation of total MoPrP signal in each fraction (from the top, fraction 1, to the bottom, fraction 10) was related to the highest value, which was taken as 100%. Similar results were obtained in three independent experiments, and panel A results were also obtained with infected GT1-7 cells. (C) An aliquot of the fractions of panel A was used in a GM1 dot blot assay (see Materials and Methods). GM1 was concentrated in fractions 2 to 4 prior to AmB treatment and in fractions 3 to 5 after treatment. (D) Samples from panel A were used for this panel, but similar results were obtained with the other gradients used in this work. For each fraction, the sucrose and protein concentrations were determined, respectively, by reflectometry and BCA protein assay. No significant modification of either profile was noted after AmB treatment.
Similar articles
- Effect of amphotericin B on wild-type and mutated prion proteins in cultured cells: putative mechanism of action in transmissible spongiform encephalopathies.
Mangé A, Milhavet O, McMahon HE, Casanova D, Lehmann S. Mangé A, et al. J Neurochem. 2000 Feb;74(2):754-62. doi: 10.1046/j.1471-4159.2000.740754.x. J Neurochem. 2000. PMID: 10646527 - Effects of new amphotericin analogues on the scrapie isoform of the prion protein.
Soler L, Caffrey P, McMahon HE. Soler L, et al. Biochim Biophys Acta. 2008 Oct;1780(10):1162-7. doi: 10.1016/j.bbagen.2008.07.005. Epub 2008 Jul 18. Biochim Biophys Acta. 2008. PMID: 18691635 - Effect of Congo red on wild-type and mutated prion proteins in cultured cells.
Milhavet O, Mangé A, Casanova D, Lehmann S. Milhavet O, et al. J Neurochem. 2000 Jan;74(1):222-30. doi: 10.1046/j.1471-4159.2000.0740222.x. J Neurochem. 2000. PMID: 10617123 - Prion encephalopathies of animals and humans.
Prusiner SB. Prusiner SB. Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review. - Insights into Mechanisms of Transmission and Pathogenesis from Transgenic Mouse Models of Prion Diseases.
Moreno JA, Telling GC. Moreno JA, et al. Methods Mol Biol. 2017;1658:219-252. doi: 10.1007/978-1-4939-7244-9_16. Methods Mol Biol. 2017. PMID: 28861793 Free PMC article. Review.
Cited by
- Differentiated cultures of an immortalized human neural progenitor cell line do not replicate prions despite PrPC overexpression.
Slota JA, Wang X, Lusansky D, Medina SJ, Booth SA. Slota JA, et al. Prion. 2023 Dec;17(1):116-132. doi: 10.1080/19336896.2023.2206315. Prion. 2023. PMID: 37131335 Free PMC article. - Neurodegeneration, Mitochondria, and Antibiotics.
Suárez-Rivero JM, López-Pérez J, Muela-Zarzuela I, Pastor-Maldonado C, Cilleros-Holgado P, Gómez-Fernández D, Álvarez-Córdoba M, Munuera-Cabeza M, Talaverón-Rey M, Povea-Cabello S, Suárez-Carrillo A, Piñero-Pérez R, Reche-López D, Romero-Domínguez JM, Sánchez-Alcázar JA. Suárez-Rivero JM, et al. Metabolites. 2023 Mar 12;13(3):416. doi: 10.3390/metabo13030416. Metabolites. 2023. PMID: 36984858 Free PMC article. Review. - What is the role of lipids in prion conversion and disease?
Alves Conceição C, Assis de Lemos G, Barros CA, Vieira TCRG. Alves Conceição C, et al. Front Mol Neurosci. 2023 Jan 10;15:1032541. doi: 10.3389/fnmol.2022.1032541. eCollection 2022. Front Mol Neurosci. 2023. PMID: 36704327 Free PMC article. Review. - Prion therapeutics: Lessons from the past.
Shim KH, Sharma N, An SSA. Shim KH, et al. Prion. 2022 Dec;16(1):265-294. doi: 10.1080/19336896.2022.2153551. Prion. 2022. PMID: 36515657 Free PMC article. Review. - Polyene Antibiotics Physical Chemistry and Their Effect on Lipid Membranes; Impacting Biological Processes and Medical Applications.
Haro-Reyes T, Díaz-Peralta L, Galván-Hernández A, Rodríguez-López A, Rodríguez-Fragoso L, Ortega-Blake I. Haro-Reyes T, et al. Membranes (Basel). 2022 Jun 30;12(7):681. doi: 10.3390/membranes12070681. Membranes (Basel). 2022. PMID: 35877884 Free PMC article. Review.
References
- Adjou K T, Deslys J P, Lasmézas C, Demaimay R, Dormont D. Hypothèse sur les mécanismes d'action de l'amphotéricine B et de ses dérivés dans les encéphalopathies subaigües spongiformes transmissibles. Medecine/Science. 1997;13:892–896.
- Boesze-Battaglia K, Schimmel R. Cell membrane lipid composition and distribution: implications for cell function and lessons learned from photoreceptors and platelets. J Exp Biol. 1997;200:2927–2936. - PubMed
- Borchelt D R, Rogers M, Stahl N, Telling G, Prusiner S B. Release of the cellular prion protein from cultured cells after loss of its glycoinositol phospholipid anchor. Glycobiology. 1993;3:319–329. - PubMed
- Borchelt D R, Taraboulos A, Prusiner S B. Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J Biol Chem. 1992;267:16188–16199. - PubMed
- Brown D A, Rose J K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials