An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor - PubMed (original) (raw)
An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor
J L Blond et al. J Virol. 2000 Apr.
Abstract
A new human endogenous retrovirus (HERV) family, termed HERV-W, was recently described (J.-L. Blond, F. Besème, L. Duret, O. Bouton, F. Bedin, H. Perron, B. Mandrand, and F. Mallet, J. Virol. 73:1175-1185, 1999). HERV-W mRNAs were found to be specifically expressed in placenta cells, and an env cDNA containing a complete open reading frame was recovered. In cell-cell fusion assays, we demonstrate here that the product of the HERV-W env gene is a highly fusogenic membrane glycoprotein. Transfection of an HERV-W Env expression vector in a panel of cell lines derived from different species resulted in formation of syncytia in primate and pig cells upon interaction with the type D mammalian retrovirus receptor. Moreover, envelope glycoproteins encoded by HERV-W were specifically detected in placenta cells, suggesting that they may play a physiological role during pregnancy and placenta formation.
Figures
FIG. 1
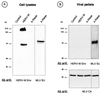
Detection of envelope glycoprotein in HERV-W Env-transfected cells. TELCeB6 cells were transfected with plasmids expressing either the HERV-W env cDNA in the antisense (Control) or positive (HERV-W) orientation or a hyperfusogenic mutant amphotropic MLV envelope glycoprotein (A-Rless). Two days later, the Env-transfected cells were lysed and their supernatants were harvested, filtered, and ultracentrifuged at 150,000 × g to pellet the viral particles. Immunoblots of cell lysates (A) and viral pellets (B) were probed with antibodies (Ab) against HERV-W Env, MLV SU, or MLV CA protein, as indicated. The positions of molecular mass markers are shown to the left of the gels (in kilodaltons).
FIG. 2
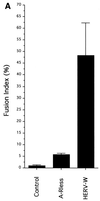
Formation of syncytia by HERV-W envelope glycoprotein. TELac2 cells, derived from TE671 human rhabdomyosarcoma cells and constitutively expressing a nuclear β-galactosidase, were transfected with plasmids expressing either the HERV-W env cDNA in the positive (HERV-W) or antisense (Control) orientation or a hyperfusogenic mutant amphotropic MLV envelope glycoprotein (A-Rless). Transfected cells were overlaid with HeLa indicator cells. The determination of the fusion activity of the transfected envelope glycoproteins was performed after 36 h of coculture. (A) Results are expressed as percentages of the fusion indices (means ± standard deviations; n = 5). (B) Cocultures were stained with X-Gal substrate to reveal β-galactosidase activity and to visualize the nuclei of the producer cells (arrows) and then with May-Grünwald and Giemsa solutions. Magnification, ×250.
FIG. 2
Formation of syncytia by HERV-W envelope glycoprotein. TELac2 cells, derived from TE671 human rhabdomyosarcoma cells and constitutively expressing a nuclear β-galactosidase, were transfected with plasmids expressing either the HERV-W env cDNA in the positive (HERV-W) or antisense (Control) orientation or a hyperfusogenic mutant amphotropic MLV envelope glycoprotein (A-Rless). Transfected cells were overlaid with HeLa indicator cells. The determination of the fusion activity of the transfected envelope glycoproteins was performed after 36 h of coculture. (A) Results are expressed as percentages of the fusion indices (means ± standard deviations; n = 5). (B) Cocultures were stained with X-Gal substrate to reveal β-galactosidase activity and to visualize the nuclei of the producer cells (arrows) and then with May-Grünwald and Giemsa solutions. Magnification, ×250.
FIG. 3
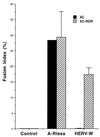
Cell-cell fusion assays in RDR-transfected XC cells. A plasmid encoding RDR, the human type D mammalian retrovirus receptor, was expressed in XC rat cells. The A-Rless or HERV-W fusogenic envelope glycoprotein was transiently expressed in RDR-transfected XC cells (hatched bars) as well as in parental XC cells (black bars). The results are expressed as percentages of the fusion indices (means ± standard deviations n = 3).
FIG. 4
In situ detection of HERV-W envelope glycoprotein in tissue sections. The tissue sections originated from human tissue donors' 13-week placenta, uterus, and small intestine. Slides were labeled (A) or not (B) with the 6A2B2 monoclonal antibody and revealed by immunoperoxidase staining and hematoxylin counterstaining. Magnification, ×100.
Similar articles
- The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors.
Lavillette D, Marin M, Ruggieri A, Mallet F, Cosset FL, Kabat D. Lavillette D, et al. J Virol. 2002 Jul;76(13):6442-52. doi: 10.1128/jvi.76.13.6442-6452.2002. J Virol. 2002. PMID: 12050356 Free PMC article. - Envelope gene of the human endogenous retrovirus HERV-W encodes a functional retrovirus envelope.
An DS, Xie Ym, Chen IS. An DS, et al. J Virol. 2001 Apr;75(7):3488-9. doi: 10.1128/JVI.75.7.3488-3489.2001. J Virol. 2001. PMID: 11238877 Free PMC article. - The envelope glycoprotein of human endogenous retrovirus HERV-W induces cellular resistance to spleen necrosis virus.
Ponferrada VG, Mauck BS, Wooley DP. Ponferrada VG, et al. Arch Virol. 2003 Apr;148(4):659-75. doi: 10.1007/s00705-002-0960-x. Arch Virol. 2003. PMID: 12664292 - Expression and functions of human endogenous retroviruses in the placenta: an update.
Muir A, Lever A, Moffett A. Muir A, et al. Placenta. 2004 Apr;25 Suppl A:S16-25. doi: 10.1016/j.placenta.2004.01.012. Placenta. 2004. PMID: 15033302 Review. - The important biological roles of Syncytin-1 of human endogenous retrovirus W (HERV-W) and Syncytin-2 of HERV-FRD in the human placenta development.
Gholami Barzoki M, Shatizadeh Malekshahi S, Heydarifard Z, Mahmodi MJ, Soltanghoraee H. Gholami Barzoki M, et al. Mol Biol Rep. 2023 Sep;50(9):7901-7907. doi: 10.1007/s11033-023-08658-0. Epub 2023 Jul 8. Mol Biol Rep. 2023. PMID: 37421503 Review.
Cited by
- Ancestral capture of syncytin-Car1, a fusogenic endogenous retroviral envelope gene involved in placentation and conserved in Carnivora.
Cornelis G, Heidmann O, Bernard-Stoecklin S, Reynaud K, Véron G, Mulot B, Dupressoir A, Heidmann T. Cornelis G, et al. Proc Natl Acad Sci U S A. 2012 Feb 14;109(7):E432-41. doi: 10.1073/pnas.1115346109. Epub 2012 Jan 17. Proc Natl Acad Sci U S A. 2012. PMID: 22308384 Free PMC article. - A novel human endogenous retroviral protein inhibits cell-cell fusion.
Sugimoto J, Sugimoto M, Bernstein H, Jinno Y, Schust D. Sugimoto J, et al. Sci Rep. 2013;3:1462. doi: 10.1038/srep01462. Sci Rep. 2013. PMID: 23492904 Free PMC article. - Early Syncytialization of the Ovine Placenta Revisited.
Seo H, Bazer FW, Johnson GA. Seo H, et al. Results Probl Cell Differ. 2024;71:127-142. doi: 10.1007/978-3-031-37936-9_7. Results Probl Cell Differ. 2024. PMID: 37996676 Review. - Unique Structure and Distinctive Properties of the Ancient and Ubiquitous Gamma-Type Envelope Glycoprotein.
Hogan V, Johnson WE. Hogan V, et al. Viruses. 2023 Jan 18;15(2):274. doi: 10.3390/v15020274. Viruses. 2023. PMID: 36851488 Free PMC article. Review. - Could the Human Endogenous Retrovirus-Derived Syncytialization Inhibitor, Suppressyn, Limit Heterotypic Cell Fusion Events in the Decidua?
Sugimoto J, Choi S, Sheridan MA, Koh I, Kudo Y, Schust DJ. Sugimoto J, et al. Int J Mol Sci. 2021 Sep 23;22(19):10259. doi: 10.3390/ijms221910259. Int J Mol Sci. 2021. PMID: 34638599 Free PMC article.
References
- Bateman, A., F. Bullough, S. Murphy, L. Emiliusen, D. Lavillette, F.-L. Cosset, R. Cattaneo, S. J. Russell, and R. Vile. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumour growth. Cancer Res., in press. - PubMed
- Berlioz-Torrent C, Shacklett B L, Erdtmann L, Delamarre L, Bouchaert I, Sonigo P, Dokhelar M C, Benarous R. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J Virol. 1999;73:1350–1361. - PMC - PubMed
- Boeke J D, Stoye J P. Retrotransposons, endogenous retroviruses, and the evolution of retroelements. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 343–435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases