The group II metabotropic glutamate receptor agonist, DCG-IV, alleviates akinesia following intranigral or intraventricular administration in the reserpine-treated rat - PubMed (original) (raw)
The group II metabotropic glutamate receptor agonist, DCG-IV, alleviates akinesia following intranigral or intraventricular administration in the reserpine-treated rat
L Dawson et al. Br J Pharmacol. 2000 Feb.
Abstract
1. This study examined whether activation of group II metabotropic glutamate (mGlu) receptors in the substantia nigra pars reticulata (SNr) could reverse akinesia in a rodent model of Parkinson's disease (PD). 2. Male Sprague Dawley rats, stereotaxically cannulated above either the SNr or third ventricle, were rendered akinetic by injection of reserpine (5 mg kg-1 s.c.). Eighteen hours later, the rotational behaviour induced by unilateral injection of the group II mGlu receptor agonist, (2S,2'R,3'R)-2-(2',3'-dicarboxycyclopropyl)glycine (DCG-IV), was examined. 3. Following intranigral injection, DCG-IV (0.125-0.75 nmol in 0.1 microliter) produced a dose-dependent increase in net contraversive rotations (n = 6-8 animals per dose), reaching a maximum of 395 +/- 51 rotations 60 min-1 after 0.75 nmol. The effects of DCG-IV (0.5 nmol) were inhibited by 63.0 +/- 9.0% following 30 min pre-treatment with the group II mGlu receptor antagonist, (2S)-alpha-ethylglutamic acid (EGLU; 100 nmol in 0.2 microliter; n = 6). 4. Following intraventricular injection, DCG-IV (0.125-1.5 nmol in 2 microliters) produced a dose-dependent increase in bilateral locomotor activity (n = 6-7 animals per dose), reaching a maximum of 180 +/- 21 locomotor units 30 min-1 after 0.5 nmol. Pre-treatment with EGLU (200 nmol in 2 microliters) inhibited the effects of DCG-IV (0.5 nmol) by 68.2 +/- 12.3% (n = 5). 5. These data show that activation of group II mGlu receptors in the SNr provides relief of akinesia in the reserpinized rat model of PD. The reversal seen following intraventricular administration supports the likely therapeutic benefit of systemically-active group II mGlu receptor agonists in PD.
Figures
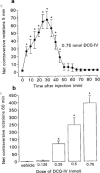
Figure 1
(a) Time-course of locomotor activity induced by a maximally-effective dose of the group II mGlu receptor agonist, DCG-IV (0.75 nmol in 0.1 μl) and (b) Dose-related locomotor effects of DCG-IV (0.125–0.75 nmol in 0.1 μl) or vehicle (0.1 μl PBS), following unilateral injection into the SNr of the reserpine-treated rat. Values represent mean±s.e.mean (_n_=6–8 animals per dose). *Indicates a significant difference compared to (a) baseline activity or (b) the previous dose (1-way ANOVA, P<0.05 in all cases).
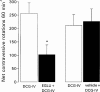
Figure 2
Comparison of locomotor activity induced by intranigral injection of the group II mGlu receptor agonist, DCG-IV (0.5 nmol in 0.1 μl) prior to (open bars) and 30 min following (closed bars) treatment with the group II mGlu receptor antagonist, EGLU (100 nmol in 0.1 μl) or vehicle (0.1 μl of 0.3 m
M
NaOH in PBS). Data represent mean±s.e.mean (_n_=6 animals per treatment). *Indicates a significant difference between pre-treatment and post-treatment responses to DCG-IV (paired _t_-test, P<0.05).
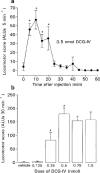
Figure 3
(a) Time-course of locomotor activity induced by a maximally-effective dose of DCG-IV (0.5 nmol in 2 μl) and (b) dose-related locomotor effects of DCG-IV (0.125–1.5 nmol in 2 μl) or vehicle (2 μl of PBS), following intraventricular injection in the reserpine-treated rat. Values represent mean±s.e.mean (_n_=6–7 animals per dose). *Indicates a significant difference compared to (a) baseline activity or (b) the previous dose (1-way ANOVA, P<0.05 in all cases).
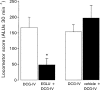
Figure 4
Comparison of locomotor activity induced by intraventricular injection of the group II mGlu receptor agonist, DCG-IV (0.5 nmol in 2 μl) prior to (open bars) and 30 min following (closed bars) treatment with the group II mGlu receptor antagonist, EGLU (200 nmol in 2 μl) or vehicle (2 μl of 0.3 m
M
NaOH in PBS). Data represent mean±s.e.mean (_n_=5 animals per treatment). *Indicates a significant difference between pre-treatment and post-treatment responses to DCG-IV (paired _t_-test, P<0.05).
Similar articles
- Activation of group III metabotropic glutamate receptors in selected regions of the basal ganglia alleviates akinesia in the reserpine-treated rat.
MacInnes N, Messenger MJ, Duty S. MacInnes N, et al. Br J Pharmacol. 2004 Jan;141(1):15-22. doi: 10.1038/sj.bjp.0705566. Epub 2003 Nov 3. Br J Pharmacol. 2004. PMID: 14597605 Free PMC article. - Neuroprotective effects of group II metabotropic glutamate receptor agonist DCG-IV on hippocampal neurons in transient forebrain ischemia.
Yoshioka H, Sugita M, Kinouchi H. Yoshioka H, et al. Neurosci Lett. 2009 Sep 25;461(3):266-70. doi: 10.1016/j.neulet.2009.06.056. Epub 2009 Jun 21. Neurosci Lett. 2009. PMID: 19549561 - [Excitatory amino acids and neuronal death].
Shinozaki H. Shinozaki H. No To Hattatsu. 1995 Mar;27(2):104-12. No To Hattatsu. 1995. PMID: 7727152 Review. Japanese. - Therapeutic potential of targeting group III metabotropic glutamate receptors in the treatment of Parkinson's disease.
Duty S. Duty S. Br J Pharmacol. 2010 Sep;161(2):271-87. doi: 10.1111/j.1476-5381.2010.00882.x. Br J Pharmacol. 2010. PMID: 20735415 Free PMC article. Review.
Cited by
- Calcium signaling in neurodegeneration.
Marambaud P, Dreses-Werringloer U, Vingtdeux V. Marambaud P, et al. Mol Neurodegener. 2009 May 6;4:20. doi: 10.1186/1750-1326-4-20. Mol Neurodegener. 2009. PMID: 19419557 Free PMC article. - Beneficial effects of natural phenolics on levodopa methylation and oxidative neurodegeneration.
Kang KS, Yamabe N, Wen Y, Fukui M, Zhu BT. Kang KS, et al. Brain Res. 2013 Feb 25;1497:1-14. doi: 10.1016/j.brainres.2012.11.043. Epub 2012 Dec 1. Brain Res. 2013. PMID: 23206800 Free PMC article. - Therapeutic potential of targeting glutamate receptors in Parkinson's disease.
Finlay C, Duty S. Finlay C, et al. J Neural Transm (Vienna). 2014 Aug;121(8):861-80. doi: 10.1007/s00702-014-1176-4. Epub 2014 Feb 21. J Neural Transm (Vienna). 2014. PMID: 24557498 Review. - Enhanced sensitivity to group II mGlu receptor activation at corticostriatal synapses in mice lacking the familial parkinsonism-linked genes PINK1 or Parkin.
Martella G, Platania P, Vita D, Sciamanna G, Cuomo D, Tassone A, Tscherter A, Kitada T, Bonsi P, Shen J, Pisani A. Martella G, et al. Exp Neurol. 2009 Feb;215(2):388-96. doi: 10.1016/j.expneurol.2008.11.001. Epub 2008 Nov 21. Exp Neurol. 2009. PMID: 19071114 Free PMC article. - Animal models of Parkinson's disease: a source of novel treatments and clues to the cause of the disease.
Duty S, Jenner P. Duty S, et al. Br J Pharmacol. 2011 Oct;164(4):1357-91. doi: 10.1111/j.1476-5381.2011.01426.x. Br J Pharmacol. 2011. PMID: 21486284 Free PMC article. Review.
References
- BATTAGLIA G., MONN J.A., SCHOEPP D.D. In vivo inhibition of veratridine-evoked release of striatal excitatory amino acids by the group II metabotropic glutamate receptor agonist LY35470 in rats. Neurosci. Lett. 1997;229:161–164. - PubMed
- BENAZZOUZ A., GROSS C., FEGER J., BORAUD T., BIOULAC B. Reversal of rigidity and improvement of motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur. J. Neurosci. 1993;5:382–389. - PubMed
- BERGMAN H., WICHMANN T., DELONG M.R. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1346–1348. - PubMed
- BROTCHIE J.M., CROSSMAN A.R. D-[3H]Aspartate and [14C]GABA uptake in the basal ganglia of rats following lesions in the subthalamic region suggest a role for excitatory amino acids but not GABA-mediated transmission in subthalamic nucleus efferents. Exp. Neurol. 1992;113:171–181. - PubMed
- CALABRESI P., CENTONZE D., PISANI A., BERNARDI G. Metabotropic glutamate receptors and cell-type-specific vulnerability in the rat striatum: implication for ischaemia and Huntington's disease. Exp. Neurol. 1999;158:97–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous