Gene dosage affects the cardiac and brain phenotype in nonmuscle myosin II-B-depleted mice - PubMed (original) (raw)
. 2000 Mar;105(5):663-71.
doi: 10.1172/JCI8199.
H K Hwang, Y Hara, K Takeda, S Kawamoto, A N Tullio, Z X Yu, V J Ferrans, N Tresser, A Grinberg, Y A Preston, R S Adelstein
Affiliations
- PMID: 10712438
- PMCID: PMC289177
- DOI: 10.1172/JCI8199
Gene dosage affects the cardiac and brain phenotype in nonmuscle myosin II-B-depleted mice
D Uren et al. J Clin Invest. 2000 Mar.
Abstract
Complete ablation of nonmuscle myosin heavy chain II-B (NMHC-B) in mice resulted in cardiac and brain defects that were lethal during embryonic development or on the day of birth. In this paper, we report on the generation of mice with decreased amounts of NMHC-B. First, we generated B(DeltaI)/B(DeltaI) mice by replacing a neural-specific alternative exon with the PGK-Neo cassette. This resulted in decreased amounts of NMHC-B in all tissues, including a decrease of 88% in the heart and 65% in the brain compared with B(+)/B(+) tissues. B(DeltaI)/B(DeltaI) mice developed cardiac myocyte hypertrophy between 7 months and 11 months of age, at which time they reexpressed the cardiac beta-MHC. Serial sections of B(DeltaI)/B(DeltaI) brains showed abnormalities in neural cell migration and adhesion in the ventricular wall. Crossing B(DeltaI)/B(DeltaI) with B(+)/B(-) mice generated B(DeltaI)/B(-) mice, which showed a further decrease of approximately 55% in NMHC-B in the heart and brain compared with B(DeltaI)/B(DeltaI) mice. Five of 8 B(DeltaI)/B(-) mice were born with a membranous ventricular septal defect. Moreover, 5 of 5 B(DeltaI)/B(-) mice developed myocyte hypertrophy by 1 month; B(DeltaI)/B(-) mice also reexpressed the cardiac beta-MHC. More than 60% of B(DeltaI)/B(-) mice developed overt hydrocephalus and showed more severe defects in neural cell migration and adhesion than did B(DeltaI)/B(DeltaI) mice. These data on B(DeltaI)/B(DeltaI) and B(DeltaI)/B(-) mice demonstrate a gene dosage effect of the amount of NMHC-B on the severity and time of onset of the defects in the heart and brain.
Figures
Figure 1
Generation and genomic analysis of BΔΙ/BΔΙ mice. (a) Genomic map of the NMHC-B locus surrounding the N30 neural-specific alternative exon. E5 and E6 are constitutive exons encoding part of the ATP-binding region of NMHC-B. The _Hin_dII-_Eco_RI fragment, which includes the N30 exon, was replaced by the PGK-Neo gene. The PGK-Neo cassette (NEO) and the herpes simplex thymidine kinase gene (TK) are indicated by boxes. Arrows above the boxes indicate the direction of transcription. Some restriction sites for enzymes marked with an asterisk are not shown. Exons are not drawn to scale. (b) Southern blot analysis of genomic DNA isolated from mouse tails. _Nco_I was used to generate a 10.7-kb fragment indicating the wild-type allele and a 6.6-kb fragment indicating the mutant allele. The probe used for Southern analysis is shown below the mutant allele in a.
Figure 2
Immunoblot analysis of tissues from E14.5 and 11-month-old mice. (a) Comparison of NMHC-B in various tissues from B+/B+, B+/BΔΙ, and BΔΙ/BΔΙ mice at E14.5. NMHC-B levels are decreased by 65 ± 7% in the brain (n = 4) and by 88 ± 4% in the heart (n = 5) of BΔΙ/BΔΙ mice compared with B+/B+ littermates. The decrease in the lung sample shown is 64%. (b) Reexpression of the fetal gene program showing a more than 5-fold increase in the cardiac β-MHC in 2 different BΔΙ/BΔΙ mice at 11 months of age. Two different loadings are shown for each sample. The immunoblots were overexposed to permit comparison with B+/B+ samples. Quantitation was normalized using an antibody to actin (shown) and GAPDH (not shown) after B+/B+ samples.
Figure 3
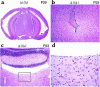
Myocyte size measurements and histological sections of B+/B+ and BΔΙ/BΔΙ mouse hearts. (a) Plot of transverse diameters of cardiac myocytes vs. age of mice (n = 3 for each point; P < 0.05 at 11 months of age). (b) Representative sections of a B+/B+ mouse heart (left) and a BΔΙ/BΔΙ mouse heart (right) stained with periodic acid–methenamine silver, demonstrating cardiac hypertrophy at age 11 months. Bar, 10 μm.
Figure 4
Brain defects in BΔΙ/BΔΙ mice at P29 and P23. (a) Enlargement of lateral ventricles and destruction of the ventricular and subventricular structure in a P29 BΔΙ/BΔΙ mouse. (b) Stenosis of the cerebral aqueduct with loss of the ependymal cells, and protrusion of a nerve fiber bundle in a BΔΙ/BΔΙ mouse at P23. (c) Loss of the ependymal cells and misplaced large neurons at the level of the fourth ventricle in a BΔΙ/BΔΙ mouse at P23. (d) Enlargement of the boxed area shown in c. A number of misplaced large neurons are present (arrows). Coronal sections; H&E stain.
Figure 5
Immunoblot analysis of tissues from P0 and P23 mice. (a) Comparison of NMHC-B from BΔΙ/B–, BΔΙ/BΔΙ, and B+/B– mice at P0. Two different amounts were loaded for each sample. Quantitation was normalized using an antibody to actin. NMHC-B was decreased by 56 ± 9% (n = 4) and 55 ± 5% (n = 4) in brain and heart, respectively, compared with BΔΙ/BΔΙ mice. The decrease in the lung sample shown is 25%. (b) Reexpression of the fetal gene program in the hearts of 2 BΔΙ/B– mice at P23 shows an approximately 40-fold increase in upregulation of the cardiac β-MHC compared with B+/B+ mice. Two different loadings are shown for each sample. Quantitation was normalized using an antibody to GAPDH.
Figure 6
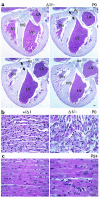
Histological sections of BΔΙ/B– and B+/BΔΙ mouse hearts. (a) Four selected serial sections, anterior to posterior (clockwise from upper left), demonstrating the defect (arrowheads) in the membranous region of the ventricular septum in a P0 BΔΙ/B– mouse heart. Ao, aorta; IVS, interventricular septum; LA, left atrium; LV, left ventricle; RV, right ventricle. H&E stain. Bar, 200 μm. (b) Normal cardiac histology in a B+/BΔΙ mouse (left) and area of myocyte disarray and hyperchromatic nuclei in a BΔΙ/B– mouse (right). (c) Hypertrophied myocytes from a P24 BΔΙ/B– mouse (right) and myocytes from a B+/BΔΙ littermate (left). H&E stain. Bar, 20 μm.
Figure 7
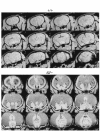
Magnetic resonance microscopy images of brain from a normal wild-type mouse (top) and a P24 BΔΙ/B– mouse (bottom). The images in the bottom panel show massive enlargement of the lateral ventricles and thinning of the cerebral cortex (arrow) due to hydrocephalus.
Figure 8
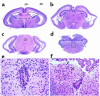
Brain defects in BΔΙ/B– mice at P0. (a–d) Low-magnification view of coronal sections from anterior to posterior. The lateral ventricles are expanded and the ventricular and subventricular structures of the cerebral cortex are destroyed (a–c), but the fourth ventricle is not expanded (d). The choroidal plexus looks normal (a, b, and d). (e) Enlargement of the boxed area shown in c showing stenosis and deformity of the cerebral aqueduct and the asymmetric development of the neuroepithelial cells. (f) Enlargement of the boxed area shown in d, showing loss of ependymal cells and the abnormal protrusion of a mass of cells containing large neurons into the fourth ventricle. The misplaced large neurons (arrows) are present in abundance. The ependymal cell layer is indicated (arrowhead). H&E stain.
Similar articles
- In vivo studies on nonmuscle myosin II expression and function in heart development.
Ma X, Adelstein RS. Ma X, et al. Front Biosci (Landmark Ed). 2012 Jan 1;17(2):545-55. doi: 10.2741/3942. Front Biosci (Landmark Ed). 2012. PMID: 22201759 Free PMC article. Review. - Ablation and mutation of nonmuscle myosin heavy chain II-B results in a defect in cardiac myocyte cytokinesis.
Takeda K, Kishi H, Ma X, Yu ZX, Adelstein RS. Takeda K, et al. Circ Res. 2003 Aug 22;93(4):330-7. doi: 10.1161/01.RES.0000089256.00309.CB. Epub 2003 Jul 31. Circ Res. 2003. PMID: 12893741 - Nonmuscle myosin II-B is required for normal development of the mouse heart.
Tullio AN, Accili D, Ferrans VJ, Yu ZX, Takeda K, Grinberg A, Westphal H, Preston YA, Adelstein RS. Tullio AN, et al. Proc Natl Acad Sci U S A. 1997 Nov 11;94(23):12407-12. doi: 10.1073/pnas.94.23.12407. Proc Natl Acad Sci U S A. 1997. PMID: 9356462 Free PMC article. - Structural abnormalities develop in the brain after ablation of the gene encoding nonmuscle myosin II-B heavy chain.
Tullio AN, Bridgman PC, Tresser NJ, Chan CC, Conti MA, Adelstein RS, Hara Y. Tullio AN, et al. J Comp Neurol. 2001 Apr 23;433(1):62-74. doi: 10.1002/cne.1125. J Comp Neurol. 2001. PMID: 11283949 - Inactivation of myosin heavy chain genes in the mouse: diverse and unexpected phenotypes.
Allen DL, Harrison BC, Leinwand LA. Allen DL, et al. Microsc Res Tech. 2000 Sep 15;50(6):492-9. doi: 10.1002/1097-0029(20000915)50:6<492::AID-JEMT6>3.0.CO;2-J. Microsc Res Tech. 2000. PMID: 10998638 Review.
Cited by
- Conventional and Non-Conventional Roles of Non-Muscle Myosin II-Actin in Neuronal Development and Degeneration.
Javier-Torrent M, Saura CA. Javier-Torrent M, et al. Cells. 2020 Aug 19;9(9):1926. doi: 10.3390/cells9091926. Cells. 2020. PMID: 32825197 Free PMC article. Review. - Function of the neuron-specific alternatively spliced isoforms of nonmuscle myosin II-B during mouse brain development.
Ma X, Kawamoto S, Uribe J, Adelstein RS. Ma X, et al. Mol Biol Cell. 2006 May;17(5):2138-49. doi: 10.1091/mbc.e05-10-0997. Epub 2006 Feb 15. Mol Biol Cell. 2006. PMID: 16481398 Free PMC article. - S100P dissociates myosin IIA filaments and focal adhesion sites to reduce cell adhesion and enhance cell migration.
Du M, Wang G, Ismail TM, Gross S, Fernig DG, Barraclough R, Rudland PS. Du M, et al. J Biol Chem. 2012 May 4;287(19):15330-44. doi: 10.1074/jbc.M112.349787. Epub 2012 Mar 6. J Biol Chem. 2012. PMID: 22399300 Free PMC article. - Ablation of nonmuscle myosin II-B and II-C reveals a role for nonmuscle myosin II in cardiac myocyte karyokinesis.
Ma X, Jana SS, Conti MA, Kawamoto S, Claycomb WC, Adelstein RS. Ma X, et al. Mol Biol Cell. 2010 Nov 15;21(22):3952-62. doi: 10.1091/mbc.E10-04-0293. Epub 2010 Sep 22. Mol Biol Cell. 2010. PMID: 20861308 Free PMC article. - In vivo studies on nonmuscle myosin II expression and function in heart development.
Ma X, Adelstein RS. Ma X, et al. Front Biosci (Landmark Ed). 2012 Jan 1;17(2):545-55. doi: 10.2741/3942. Front Biosci (Landmark Ed). 2012. PMID: 22201759 Free PMC article. Review.
References
- Watkins H, Seidman JG, Seidman CE. Familial hypertrophic cardiomyopathy: a genetic model of cardiac hypertrophy. Hum Mol Genet. 1995;4:1721–1727. - PubMed
- Olson TM, Michels VV, Thibodeau SN, Tai Y-S, Keating MT. Actin mutations in dilated cardiomyopathy, a heritable form of heart failure. Science. 1998;280:750–752. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials