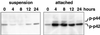The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum removal - PubMed (original) (raw)
The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum removal
M Le Gall et al. Mol Biol Cell. 2000 Mar.
Free PMC article
Abstract
Anchorage removal like growth factor removal induces apoptosis. In the present study we have characterized signaling pathways that can prevent this cell death using a highly growth factor- and anchorage-dependent line of lung fibroblasts (CCL39). After anchorage removal from exponentially growing cells, annexin V-FITC labeling can be detected after 8 h. Apoptosis was confirmed by analysis of sub-G1 DNA content and Western blotting of the caspase substrate poly (ADP-ribose) polymerase. Growth factor withdrawal accelerates and potentiates suspension-induced cell death. Activation of Raf-1 kinase in suspension cultures of CCL39 or Madin-Darby canine kidney cells stably expressing an estrogen-inducible activated-Raf-1 construct (DeltaRaf-1:ER) suppresses apoptosis induced by growth factor and/or anchorage removal. This protective effect appears to be mediated by the Raf, mitogen- or extracellular signal-regulated kinase kinase (MEK), and mitogen-activated protein kinase module because it is sensitive to pharmacological inhibition of MEK-1 and it can be mimicked by expression of constitutively active MEK-1 in CCL39 cells. Finally, apoptosis induced by disruption of the actin cytoskeleton with the Rho-directed toxin B (Clostridium difficile) is prevented by activation of the DeltaRaf-1:ER chimeric construct. These findings highlight the ability of p42/p44 mitogen-activated protein kinase to generate survival signals that counteract cell death induced by loss of matrix contact, cytoskeletal integrity, and extracellular mitogenic factors.
Figures
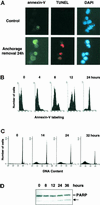
Figure 1
Anchorage removal induces apoptosis in CCL39 cells. Exponentially growing CCL39 cells were deprived of anchorage as described in MATERIALS AND METHODS. At the indicated times, cells were treated as follows. (A) Suspended cells were labeled with annexin V-FITC, fixed, and adsorbed on polylysine-coated slides before TUNEL analysis and nuclear staining with DAPI. Magnification: 630×. (B) Cells (106) were labeled with annexin V-FITC and analyzed by FACS. (C) Cells were fixed and incubated with propidium iodide, and DNA content was analyzed by FACS. (D) Immunoblot analysis of PARP was performed on total cell lysates. The arrow (here and in subsequent figures) indicates the position of the 85-kDa PARP cleavage product. Results shown are representative of at least two independent experiments.
Figure 2
Serum removal potentiates induction of apoptosis. Exponentially growing CCL39 cells were deprived of serum (top) or anchorage and serum (bottom) as described in MATERIALS AND METHODS. At the indicated times, attached cells were trypsinized and treated as suspended cells. Annexin V-FITC labeling, TUNEL analyses, nuclei staining with DAPI, and immunoblot analysis of PARP were performed as described in Figure 1. Magnification: 400×.
Figure 3
Z-Val-Ala-
d,l
-Asp-fluoromethylketone blocks apoptosis induced by anchorage and serum removal. Z-Val-Ala-
d,l
-Asp-fluoromethylketone (ZVAD) was added at the time of anchorage and serum removal. Immunoblot analysis of PARP was performed on total cell lysates from cells in suspension for the indicated times.
Figure 4
Comparison of MAP kinase activation in suspended and attached cells. Exponentially growing CCL39 cells were trypsinized, resuspended in serum-containing medium, and either placed in suspension (left), as described in MATERIALS AND METHODS, or replated in tissue culture dishes (right) for the indicated times. Immunoblot analysis of the active phosphorylated form of MAP kinase was performed on total cell lysates. p-p42, phospho-p42; p-p44, phospho-p44.
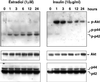
Figure 5
Raf activation in CCL39–ΔRaf-1:ER cells stimulates the MAP kinase pathway without activation of the PI3-K/Akt pathway. CCL39–ΔRaf-1:ER monolayers arrested in G0 by 18 h of serum removal were stimulated with estradiol or insulin for the indicated times. Western blot analyses were performed on whole-cell lysates using antibodies that recognize Akt or MAP kinase or their phosphorylated forms. p-Akt, phospho-Akt.
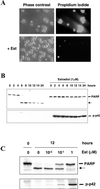
Figure 6
Inhibition of apoptosis after anchorage and serum withdrawal by Raf-1/MAP kinase activation. CCL39–ΔRaf-1:ER cells were deprived of anchorage and serum in the presence or absence of 1 μM estradiol (Est) for the indicated times. (A) Phase-contrast photomicrographs (magnification: 200×) of cells taken after 12 h (left) and propidium iodide staining (right) revealing dead, permeable cells. (B) Time course of PARP cleavage (top) and MAP kinase activation (bottom) after the detachment of cells in the presence or absence of estradiol. (C) Dose response of estradiol-induced inhibition of PARP cleavage (top) and activation of MAP kinase (bottom) after 12 h of anchorage and serum deprivation.
Figure 7
Effect of MEK-1 inhibition or stimulation on the cleavage of PARP. (A) Western blot analyses of PARP cleavage (top) and MAP kinase activation (bottom) were performed on total cell lysates from CCL39–ΔRaf-1:ER cells deprived of anchorage and serum for 12 h in the presence of 0.1 μM estradiol and 20 μM PD98059, as indicated (+). (B) Nontransfected cells (parental) or cells stably expressing constitutively active MEK-1 (MEK SS/DD) were deprived of anchorage and serum. At the indicated times, PARP cleavage (top) and MAP kinase activation (bottom) were analyzed by Western blotting.
Figure 8
Inhibition of PARP cleavage in CCL39- or MDCK-derived clones expressing ΔRaf-1:ER. (A) Western blot analyses of PARP cleavage (top) and MAP kinase activation (bottom) were performed on total cell lysates from two independent clones expressing ΔRaf-1:ER after 10 h of anchorage and serum withdrawal in the presence (+) or absence of 1 μM estradiol. Clones S18 and S19 (left) were derived from CCL39; clones Cl2 and Cl14 (right) were derived from MDCK. (B) Western blot analyses of PARP cleavage (top), Akt activation (middle), and MAP kinase activation (bottom) were performed on total cell lysates from CCL39–ΔRaf-1:ER cells (clone S18) and MDCK–ΔRaf-1:ER clone 14 (Cl14) after 10 h of anchorage and serum withdrawal in the presence (+) or absence of 1 μM estradiol or 10 μg/ml insulin (Ins).
Figure 9
Effect of MAP kinase and PI3-K inhibition on PARP cleavage. Western blot analyses of PARP cleavage (top) and MAP kinase activation (bottom) were performed on total cell lysates from CCL39–ΔRaf-1:ER cells after 20 h of suspension culture in serum-supplemented medium in the presence (+) or absence of 20 μM PD98059 or 15 μM LY294002.
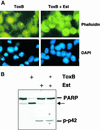
Figure 10
Antiapoptotic action of ΔRaf-1:ER after Rho inhibition. CCL39–ΔRaf-1:ER monolayers were treated with 60 pM toxin B (ToxB) in serum-free medium in the presence (+) or absence of 1 μM estradiol. (A) Polymerized actin was detected using phalloidin-FITC, and nuclei were labeled with DAPI. Magnification: 630×. (B) Western blot analyses of PARP cleavage (top) and MAP kinase activation (bottom) were performed on total cell lysates.
Similar articles
- An anchorage-dependent signal distinct from p42/44 MAP kinase activation is required for cell cycle progression.
Le Gall M, Grall D, Chambard JC, Pouysségur J, Van Obberghen-Schilling E. Le Gall M, et al. Oncogene. 1998 Sep 10;17(10):1271-7. doi: 10.1038/sj.onc.1202057. Oncogene. 1998. PMID: 9771970 - p42/p44 MAP kinase module plays a key role in the transcriptional regulation of the vascular endothelial growth factor gene in fibroblasts.
Milanini J, Viñals F, Pouysségur J, Pagès G. Milanini J, et al. J Biol Chem. 1998 Jul 17;273(29):18165-72. doi: 10.1074/jbc.273.29.18165. J Biol Chem. 1998. PMID: 9660776 - Differential abilities of the Raf family of protein kinases to abrogate cytokine dependency and prevent apoptosis in murine hematopoietic cells by a MEK1-dependent mechanism.
Hoyle PE, Moye PW, Steelman LS, Blalock WL, Franklin RA, Pearce M, Cherwinski H, Bosch E, McMahon M, McCubrey JA. Hoyle PE, et al. Leukemia. 2000 Apr;14(4):642-56. doi: 10.1038/sj.leu.2401720. Leukemia. 2000. PMID: 10764150 - Raf-MEK-Erk cascade in anoikis is controlled by Rac1 and Cdc42 via Akt.
Zugasti O, Rul W, Roux P, Peyssonnaux C, Eychene A, Franke TF, Fort P, Hibner U. Zugasti O, et al. Mol Cell Biol. 2001 Oct;21(19):6706-17. doi: 10.1128/MCB.21.19.6706-6717.2001. Mol Cell Biol. 2001. PMID: 11533257 Free PMC article. - Growth factor-induced p42/p44 MAPK nuclear translocation and retention requires both MAPK activation and neosynthesis of nuclear anchoring proteins.
Lenormand P, Brondello JM, Brunet A, Pouysségur J. Lenormand P, et al. J Cell Biol. 1998 Aug 10;142(3):625-33. doi: 10.1083/jcb.142.3.625. J Cell Biol. 1998. PMID: 9700154 Free PMC article.
Cited by
- Atypical MAP kinases - new insights and directions from amoeba.
Hadwiger JA, Aranda RG, Fatima S. Hadwiger JA, et al. J Cell Sci. 2023 Oct 15;136(20):jcs261447. doi: 10.1242/jcs.261447. Epub 2023 Oct 16. J Cell Sci. 2023. PMID: 37850857 Free PMC article. Review. - Targeted Strategies for Degradation of Key Transmembrane Proteins in Cancer.
Sakanyan V, Iradyan N, Alves de Sousa R. Sakanyan V, et al. BioTech (Basel). 2023 Sep 6;12(3):57. doi: 10.3390/biotech12030057. BioTech (Basel). 2023. PMID: 37754201 Free PMC article. Review. - Tumor cell integrin β4 and tumor stroma E-/P-selectin cooperatively regulate tumor growth in vivo.
Genduso S, Freytag V, Schetler D, Kirchner L, Schiecke A, Maar H, Wicklein D, Gebauer F, Bröker K, Stürken C, Milde-Langosch K, Oliveira-Ferrer L, Ricklefs FL, Ewald F, Wolters-Eisfeld G, Riecken K, Unrau L, Krause L, Bohnenberger H, Offermann A, Perner S, Sebens S, Lamszus K, Diehl L, Linder S, Jücker M, Schumacher U, Lange T. Genduso S, et al. J Hematol Oncol. 2023 Mar 17;16(1):23. doi: 10.1186/s13045-023-01413-9. J Hematol Oncol. 2023. PMID: 36932441 Free PMC article. - Anti-Obesity Effects of Metformin: A Scoping Review Evaluating the Feasibility of Brown Adipose Tissue as a Therapeutic Target.
Ziqubu K, Mazibuko-Mbeje SE, Mthembu SXH, Mabhida SE, Jack BU, Nyambuya TM, Nkambule BB, Basson AK, Tiano L, Dludla PV. Ziqubu K, et al. Int J Mol Sci. 2023 Jan 23;24(3):2227. doi: 10.3390/ijms24032227. Int J Mol Sci. 2023. PMID: 36768561 Free PMC article. Review. - SAR Probing of KX2-391 Provided Analogues With Juxtaposed Activity Profile Against Major Oncogenic Kinases.
Omar AM, Khayat MT, Ahmed F, Muhammad YA, Malebari AM, Ibrahim SM, Khan MI, Shah DK, Childers WE, El-Araby ME. Omar AM, et al. Front Oncol. 2022 May 20;12:879457. doi: 10.3389/fonc.2022.879457. eCollection 2022. Front Oncol. 2022. PMID: 35669422 Free PMC article.
References
- Bergmann A, Agapite J, McCall K, Steller H. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell. 1998;95:331–341. - PubMed
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
- Brunet A, Pages G, Pouysségur J. Constitutively active mutants of MAP kinase kinase (MEK1) induce growth factor-relaxation and oncogenicity when expressed in fibroblasts. Oncogene. 1994;9:3379–3387. - PubMed
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous