Photophysics and optical switching in green fluorescent protein mutants - PubMed (original) (raw)
Photophysics and optical switching in green fluorescent protein mutants
T M Creemers et al. Proc Natl Acad Sci U S A. 2000.
Abstract
We demonstrate by using low-temperature high-resolution spectroscopy that red-shifted mutants of green fluorescent protein are photo-interconverted among three conformations and are, therefore, not photostable "one-color" systems as previously believed. From our experiments we have further derived the energy-level schemes governing the interconversion among the three forms. These results have significant implications for the molecular and cell biological applications of the green fluorescent protein family; for example, in fluorescence resonant energy transfer experiments, a change in "color" on irradiation may not necessarily be due to energy transfer but can also arise from a photo-induced conversion between conformers of the excited species.
Figures
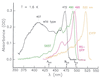
Figure 1
Absorption spectra of wild-type GFP and the red-shifted mutants S65T, RS-GFP, and EYFP at T = 1.6 K. The maxima of the absorption bands and the 0–0 transitions of the three conformers of wt-GFP are indicated.
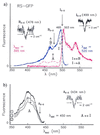
Figure 2
(a) Excitation (blue curve) and emission (red curve) spectra of RS-GFP at 1.6 K before (—) and after (---) burning into I0–0 at 499 ± 1 nm (right hole). Before burning, only the I-form is present. After burning, the vibronic bands of the B-form between 450 and 475 nm increase (blue, ---); thus, I→B. By burning into B0–0 at 476 ± 1 nm, a hole is produced (Left), and the original spectrum recovers; thus, B→I. After burning into the I0–0, the A-form is also photoinduced, which is reflected in the fluorescence spectrum. In the region from 400 to 490 nm there is no signal before burning (red, —), but there is fluorescence after burning (red, ---). (b) Excitation spectra of A with λdet = 450 nm. Optical switching between A and I occurs. (1) The A-form has been produced by burning into I0–0 at 499 ± 1 nm. (2) After burning a hole in the 0–0 transition of A at 434 ± 1 nm, the intensity of the whole A-spectrum decreases whereas that of the I-form increases (not shown). (3) After subsequent burning into I, the population of the A-form increases again.
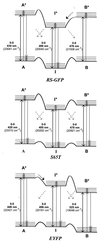
Figure 3
Energy-level diagrams of the red-shifted mutants RS-GFP (Top), S65T (Middle), and EYFP (Bottom). The forms A, I, and B photointerconvert as indicated by the diagonal arrows. The relative populations of the three forms differ for the various mutants. In RS-GFP only the I-form is populated at 1.6 K before burning; by burning into I, the A- and B-forms are photoinduced. In S65T, all forms are populated at 1.6 K, with the I-form having the lowest ground-state level. In EYFP, the most populated form is B; I and A are significantly less populated at 1.6 K. Their populations can be enhanced by burning into B.
Similar articles
- Ultra-fast excited state dynamics in green fluorescent protein: multiple states and proton transfer.
Chattoraj M, King BA, Bublitz GU, Boxer SG. Chattoraj M, et al. Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8362-7. doi: 10.1073/pnas.93.16.8362. Proc Natl Acad Sci U S A. 1996. PMID: 8710876 Free PMC article. - Red-shifted excitation mutants of the green fluorescent protein.
Delagrave S, Hawtin RE, Silva CM, Yang MM, Youvan DC. Delagrave S, et al. Biotechnology (N Y). 1995 Feb;13(2):151-4. doi: 10.1038/nbt0295-151. Biotechnology (N Y). 1995. PMID: 9634755 - Photophysics of green and red fluorescent proteins: implications for quantitative microscopy.
Subramaniam V, Hanley QS, Clayton AH, Jovin TM. Subramaniam V, et al. Methods Enzymol. 2003;360:178-201. doi: 10.1016/s0076-6879(03)60110-2. Methods Enzymol. 2003. PMID: 12622150 No abstract available. - Green and red fluorescent proteins: photo- and thermally induced dynamics probed by site-selective spectroscopy and hole burning.
Bonsma S, Purchase R, Jezowski S, Gallus J, Könz F, Völker S. Bonsma S, et al. Chemphyschem. 2005 May;6(5):838-49. doi: 10.1002/cphc.200500005. Chemphyschem. 2005. PMID: 15884066 Review. - Fluorescent proteins: maturation, photochemistry and photophysics.
Remington SJ. Remington SJ. Curr Opin Struct Biol. 2006 Dec;16(6):714-21. doi: 10.1016/j.sbi.2006.10.001. Epub 2006 Oct 24. Curr Opin Struct Biol. 2006. PMID: 17064887 Review.
Cited by
- Cathodoluminescence of green fluorescent protein exhibits the redshifted spectrum and the robustness.
Akiba K, Tamehiro K, Matsui K, Ikegami H, Minoda H. Akiba K, et al. Sci Rep. 2020 Oct 15;10(1):17342. doi: 10.1038/s41598-020-74367-4. Sci Rep. 2020. PMID: 33060754 Free PMC article. - Spectral hole burning: examples from photosynthesis.
Purchase R, Völker S. Purchase R, et al. Photosynth Res. 2009 Aug-Sep;101(2-3):245-66. doi: 10.1007/s11120-009-9484-5. Photosynth Res. 2009. PMID: 19714478 Free PMC article. Review. - Labeling proteins via hole burning of their aromatic amino acids: pressure tuning spectroscopy of BPTI.
Stübner M, Hecht C, Friedrich J. Stübner M, et al. Biophys J. 2002 Dec;83(6):3553-7. doi: 10.1016/S0006-3495(02)75355-1. Biophys J. 2002. PMID: 12496122 Free PMC article. - Super-resolution microscopy using standard fluorescent proteins in intact cells under cryo-conditions.
Kaufmann R, Schellenberger P, Seiradake E, Dobbie IM, Jones EY, Davis I, Hagen C, Grünewald K. Kaufmann R, et al. Nano Lett. 2014 Jul 9;14(7):4171-5. doi: 10.1021/nl501870p. Epub 2014 Jun 4. Nano Lett. 2014. PMID: 24884378 Free PMC article. - Light-induced flickering of DsRed provides evidence for distinct and interconvertible fluorescent states.
Malvezzi-Campeggi F, Jahnz M, Heinze KG, Dittrich P, Schwille P. Malvezzi-Campeggi F, et al. Biophys J. 2001 Sep;81(3):1776-85. doi: 10.1016/S0006-3495(01)75828-6. Biophys J. 2001. PMID: 11509387 Free PMC article.
References
- Prasher D C, Eckenrode V K, Ward W W, Prendergast F G, Cormier M J. Gene. 1992;111:229–233. - PubMed
- Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Science. 1994;263:802–805. - PubMed
- Tsien R Y. Annu Rev Biochem. 1998;67:509–544. - PubMed
- Cubitt A B, Heim R, Adams S R, Boyd A E, Gross L A, Tsien R Y. Trends Biochem Sci. 1995;20:448–455. - PubMed
- Heim R, Tsien R Y. Curr Biol. 1996;6:178–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources