Distinct roles of two conserved Staufen domains in oskar mRNA localization and translation - PubMed (original) (raw)
Distinct roles of two conserved Staufen domains in oskar mRNA localization and translation
D R Micklem et al. EMBO J. 2000.
Abstract
Drosophila Staufen protein is required for the localization of oskar mRNA to the posterior of the oocyte, the anterior anchoring of bicoid mRNA and the basal localization of prospero mRNA in dividing neuroblasts. The only regions of Staufen that have been conserved throughout animal evolution are five double-stranded (ds)RNA-binding domains (dsRBDs) and a short region within an insertion that splits dsRBD2 into two halves. dsRBDs 1, 3 and 4 bind dsRNA in vitro, but dsRBDs 2 and 5 do not, although dsRBD2 does bind dsRNA when the insertion is removed. Full-length Staufen protein lacking this insertion is able to associate with oskar mRNA and activate its translation, but fails to localize the RNA to the posterior. In contrast, Staufen lacking dsRBD5 localizes oskar mRNA normally, but does not activate its translation. Thus, dsRBD2 is required for the microtubule-dependent localization of osk mRNA, and dsRBD5 for the derepression of oskar mRNA translation, once localized. Since dsRBD5 has been shown to direct the actin-dependent localization of prospero mRNA, distinct domains of Staufen mediate microtubule- and actin-based mRNA transport.
Figures
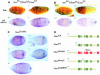
Fig. 1. A comparison of Staufen homologues from different species. (A) A diagram showing the positions of the conserved dsRBDs in D.melanogaster Staufen protein, and the percentage amino acid identity between these domains and the equivalent domains in other species. (B) A PlotSimilarity diagram of a ClustalW alignment of all six Staufen homologues. The positions of the dsRBDs are superimposed on the plot, and show that the similarity between homologues is almost entirely restricted to the dsRBDs. The arrow marks the only other region of similarity, which falls within the insertion in dsRBD2. Note that the degree of similarity shown for dsRBD1 appears lower than that for the other four dsRBDs because this domain is present in only 4/6 homologues. (C) Alignment of the conserved region in the dsRBD2 insertion. (D) An unrooted tree derived from a ClustalW alignment of dsRBDs from different proteins. Corresponding domains in the different Staufen homologues are more similar to each other than they are to any other dsRBDs, with the exception of CeStau dsRBD5, which is approximately similar to the other Stau dsRBD5s and to the third dsRBD of human TAR RNA-binding protein (Hstrbp) and Xenopus Xlrbpa (marked with asterisks). For simplicity, this diagram only includes dsRBDs described up to 1995.
Fig. 1. A comparison of Staufen homologues from different species. (A) A diagram showing the positions of the conserved dsRBDs in D.melanogaster Staufen protein, and the percentage amino acid identity between these domains and the equivalent domains in other species. (B) A PlotSimilarity diagram of a ClustalW alignment of all six Staufen homologues. The positions of the dsRBDs are superimposed on the plot, and show that the similarity between homologues is almost entirely restricted to the dsRBDs. The arrow marks the only other region of similarity, which falls within the insertion in dsRBD2. Note that the degree of similarity shown for dsRBD1 appears lower than that for the other four dsRBDs because this domain is present in only 4/6 homologues. (C) Alignment of the conserved region in the dsRBD2 insertion. (D) An unrooted tree derived from a ClustalW alignment of dsRBDs from different proteins. Corresponding domains in the different Staufen homologues are more similar to each other than they are to any other dsRBDs, with the exception of CeStau dsRBD5, which is approximately similar to the other Stau dsRBD5s and to the third dsRBD of human TAR RNA-binding protein (Hstrbp) and Xenopus Xlrbpa (marked with asterisks). For simplicity, this diagram only includes dsRBDs described up to 1995.
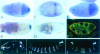
Fig. 2. The dsRBDs are the only conserved regions of Stau required for osk mRNA localization. (A) The localization of Staufen protein (i and ii) and osk mRNA (iii and iv) in stage 9 and 10 oocytes from _stau_D3 T[Staufull]/_stau_D3 females. Full-length Staufen protein expressed from the transgene localizes normally to the posterior of the oocyte, and rescues the osk mRNA posterior localization defect of a staufen null mutation. (B) The localization of Stau protein (i and ii) and osk mRNA (iii and iv) in stage 9 and 10 oocytes from _stau_D3 T[StauΔN]/_stau_D3 females. Stau protein lacking the non-conserved N-terminal 282 aa also localizes normally and rescues osk mRNA localization and anchoring. (C) (i) In wild-type egg chambers, osk mRNA shows a transient localization to the anterior of the oocyte during stage 9. (ii) In _stau_D3 T[StauDmMd]/_stau_D3 egg chambers, osk mRNA fails to accumulate at the anterior margin at stage 9, and localizes instead to the centre of the oocyte. (iii) However, the mRNA shows a normal localization at the posterior pole by stage 10. (iv) In _stau_D3 egg chambers, all osk mRNA remains anchored at the anterior of the oocyte. (v) osk mRNA shows a transient localization to a point in the centre of the oocyte, when StauDmMd is expressed in the presence of wild-type Drosophila Stau protein. (D) A diagram showing the structure of the Stau proteins encoded by the Staufull, StauΔN, StauDmMd, StauΔloop2 and StauΔdsRBD5 transgenes. The boxes indicate the positions of the dsRBDs. The short leader peptide containing the myc epitope tag is labelled in blue, Drosophila sequences in green and M.domestica sequences in red or pink.
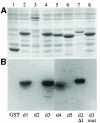
Fig. 3. dsRBDs 2 and 5 do not bind to dsRNA in vitro. (A) A Coomassie-stained SDS–PAGE gel showing the expression of the Staufen dsRBDs fused to glutathione _S_–transferase (GST). Lane 1, GST alone; lane 2, GST–dsRBD1; lane 3, GST–full-length dsRBD2; lane 4, GST–dsRBD3; lane 5, GST–dsRBD4; lane 6, GST–dsRBD5; lane 7, GST–dsRBD2 in which the large insertion has been replaced by the short loop 2 from dsRBD3; lane 8, GST–dsRBD3 containing five amino acid substitutions in residues that contact dsRNA (Ramos et al., 2000). (B) A Northwestern blot of the same samples as in (A) probed with [32P]dsRNA. The right hand side of this blot has been exposed approximately four times longer than the left to reveal the weak dsRNA-binding activity of dsRBD4. (C) A comparison of the RNA-binding faces of dsRBDs 1, 3, 4 and 5, showing the amino acids in domain 3 that are required for dsRNA binding (yellow boxes), and the identity and conservation of the amino acids in equivalent positions in the other domains (yellow). The structures of dsRBDs 1, 4 and 5 have been modelled by ‘threading’ them onto the known structure of dsRBD3 (Bycroft et al., 1995). Blue, basic residues; red, acidic; yellow, non-polar; orange, polar and uncharged. The amino acids are numbered from the first conserved residue of the domain (Ramos et al., 2000).
Fig. 3. dsRBDs 2 and 5 do not bind to dsRNA in vitro. (A) A Coomassie-stained SDS–PAGE gel showing the expression of the Staufen dsRBDs fused to glutathione _S_–transferase (GST). Lane 1, GST alone; lane 2, GST–dsRBD1; lane 3, GST–full-length dsRBD2; lane 4, GST–dsRBD3; lane 5, GST–dsRBD4; lane 6, GST–dsRBD5; lane 7, GST–dsRBD2 in which the large insertion has been replaced by the short loop 2 from dsRBD3; lane 8, GST–dsRBD3 containing five amino acid substitutions in residues that contact dsRNA (Ramos et al., 2000). (B) A Northwestern blot of the same samples as in (A) probed with [32P]dsRNA. The right hand side of this blot has been exposed approximately four times longer than the left to reveal the weak dsRNA-binding activity of dsRBD4. (C) A comparison of the RNA-binding faces of dsRBDs 1, 3, 4 and 5, showing the amino acids in domain 3 that are required for dsRNA binding (yellow boxes), and the identity and conservation of the amino acids in equivalent positions in the other domains (yellow). The structures of dsRBDs 1, 4 and 5 have been modelled by ‘threading’ them onto the known structure of dsRBD3 (Bycroft et al., 1995). Blue, basic residues; red, acidic; yellow, non-polar; orange, polar and uncharged. The amino acids are numbered from the first conserved residue of the domain (Ramos et al., 2000).
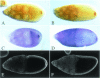
Fig. 4. The insertion in dsRBD2 is required for the posterior localization of osk mRNA. (A and B) Wild-type stage 9 and 10A egg chambers showing the normal localization of osk mRNA to the posterior of the oocyte. (C and D) _stau_D3 T[StauΔloop2]/_stau_D3 egg chambers, in which all osk mRNA remains anchored at the anterior of the oocyte. (E) A _stau_D3 T[StauΔloop2]/_stau_D3 stage 9 egg chamber, showing a small amount of osk mRNA at the posterior. (F) StauΔloop2 protein (yellow) co-localizes with osk mRNA to the anterior of the oocyte. (G) A cuticle preparation of a typical embryo from a _stau_D3;_osk_BRE– female, with a normal head and only one abdominal segment. (H and I) Typical embryos from StauΔloop2 stauD3;_osk_BRE– females, showing the loss of head structures and rescue of the abdomen (H), and the stronger symmetric bicaudal phenotype (I).
Fig. 5. dsRBD5 is required for osk mRNA translation. (A–D) Both StauΔdsRBD5 protein (A and B) and osk mRNA (C and D) localize normally to the posterior of stage 9 (A and C) and 10 oocytes (B and D). (E and F) Osk antibody stainings of wild-type (E) and _stau_D3 T[StauΔdsRBD5]/_stau_D3 oocytes (F).
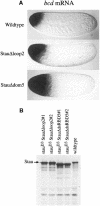
Fig. 6. dsRBD5 and the insertion in dsRBD2 are required for the anchoring of bcd mRNA. (A) bcd mRNA localization in freshly laid eggs from wild-type, _stau_D3 T[StauΔloop2]/_stau_D3 and _stau_D3 T[StauΔdsRBD5]/_stau_D3 females. (B) Western blot analysis of Stau expression in stau null mutant ovaries carrying the StauΔloop2 and StauΔdsRBD5 transgenes. Stau is expressed at higher levels than in the wild type in two independent lines of each transgene. Note that the StauΔdsRBD5 protein migrates slightly faster than the wild-type and StauΔloop2 proteins because it contains a larger deletion.
Similar articles
- A function for kinesin I in the posterior transport of oskar mRNA and Staufen protein.
Brendza RP, Serbus LR, Duffy JB, Saxton WM. Brendza RP, et al. Science. 2000 Sep 22;289(5487):2120-2. doi: 10.1126/science.289.5487.2120. Science. 2000. PMID: 11000113 Free PMC article. - Miranda couples oskar mRNA/Staufen complexes to the bicoid mRNA localization pathway.
Irion U, Adams J, Chang CW, St Johnston D. Irion U, et al. Dev Biol. 2006 Sep 15;297(2):522-33. doi: 10.1016/j.ydbio.2006.05.029. Epub 2006 May 26. Dev Biol. 2006. PMID: 16905128 - The Drosophila hnRNPA/B homolog, Hrp48, is specifically required for a distinct step in osk mRNA localization.
Huynh JR, Munro TP, Smith-Litière K, Lepesant JA, St Johnston D. Huynh JR, et al. Dev Cell. 2004 May;6(5):625-35. doi: 10.1016/s1534-5807(04)00130-3. Dev Cell. 2004. PMID: 15130488 - Localization, anchoring and translational control of oskar, gurken, bicoid and nanos mRNA during Drosophila oogenesis.
Kugler JM, Lasko P. Kugler JM, et al. Fly (Austin). 2009 Jan-Mar;3(1):15-28. doi: 10.4161/fly.3.1.7751. Epub 2009 Jan 2. Fly (Austin). 2009. PMID: 19182536 Review. - [Maternal RNA localization and RNP-complex, essential for the polarization of the oocyte in Drosophila].
Yano T. Yano T. Tanpakushitsu Kakusan Koso. 2005 Mar;50(3):246-52. Tanpakushitsu Kakusan Koso. 2005. PMID: 15773305 Review. Japanese. No abstract available.
Cited by
- Independent and coordinate trafficking of single Drosophila germ plasm mRNAs.
Little SC, Sinsimer KS, Lee JJ, Wieschaus EF, Gavis ER. Little SC, et al. Nat Cell Biol. 2015 May;17(5):558-68. doi: 10.1038/ncb3143. Epub 2015 Apr 6. Nat Cell Biol. 2015. PMID: 25848747 Free PMC article. - bicoid RNA localization requires specific binding of an endosomal sorting complex.
Irion U, St Johnston D. Irion U, et al. Nature. 2007 Feb 1;445(7127):554-8. doi: 10.1038/nature05503. Nature. 2007. PMID: 17268469 Free PMC article. - Genome-wide analysis of Staufen-associated mRNAs identifies secondary structures that confer target specificity.
Laver JD, Li X, Ancevicius K, Westwood JT, Smibert CA, Morris QD, Lipshitz HD. Laver JD, et al. Nucleic Acids Res. 2013 Nov;41(20):9438-60. doi: 10.1093/nar/gkt702. Epub 2013 Aug 13. Nucleic Acids Res. 2013. PMID: 23945942 Free PMC article. - Excessive STAU1 condensate drives mTOR translation and autophagy dysfunction in neurodegeneration.
Zhao R, Huang S, Li J, Gu A, Fu M, Hua W, Mao Y, Lei QY, Lu B, Wen W. Zhao R, et al. J Cell Biol. 2024 Aug 5;223(8):e202311127. doi: 10.1083/jcb.202311127. Epub 2024 Jun 24. J Cell Biol. 2024. PMID: 38913026 Free PMC article. - Region-specific activation of oskar mRNA translation by inhibition of Bruno-mediated repression.
Kim G, Pai CI, Sato K, Person MD, Nakamura A, Macdonald PM. Kim G, et al. PLoS Genet. 2015 Feb 27;11(2):e1004992. doi: 10.1371/journal.pgen.1004992. eCollection 2015. PLoS Genet. 2015. PMID: 25723530 Free PMC article.
References
- Adams M.D., Kerlavage, A.R., Fields, C. and Venter, J.C. (1993) 3,400 new expressed sequence tags identify diversity of transcripts in human brain. Nature Genet., 4, 256–267. - PubMed
- Bashirullah A., Cooperstock, R.L. and Lipshitz, H.D. (1998) RNA localization in development. Annu. Rev. Biochem., 67, 335–394. - PubMed
- Bertrand E., Chartrand, P., Schaefer, M., Shenoy, S.M., Singer, R.H. and Long, R.M. (1998) Localization of ASH1 mRNA particles in living yeast. Mol. Cell, 2, 437–445. - PubMed
- Broadus J. and Doe, C. (1997) Extrinsic cues, intrinsic cues and microfilaments regulate asymmetric protein localization in Drosophila neuroblasts. Curr. Biol., 7, 827–835. - PubMed
- Broadus J., Fuerstenberg, S. and Doe, C.Q. (1998) Staufen-dependent localization of prospero mRNA contributes to neuroblast daughter cell fate. Nature, 391, 792–795. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases