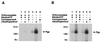Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein - PubMed (original) (raw)
Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein
Z E Sauna et al. Proc Natl Acad Sci U S A. 2000.
Abstract
P-glycoprotein (Pgp) is an ATP-dependent hydrophobic natural product anticancer drug efflux pump whose overexpression confers multidrug resistance to tumor cells. The work reported here deals with the elucidation of the energy requirement for substrate interaction with Pgp during the catalytic cycle. We show that the K(d) (412 nM) of the substrate analogue [(125)I]iodoarylazidoprazoin for Pgp is not altered by the presence of the nonhydrolyzable nucleotide 5'-adenylylimididiphosphate and vanadate (K(d) = 403 nM). Though binding of nucleotide per se does not affect interactions with the substrate, ATP hydrolysis results in a dramatic conformational change where the affinity of [(125)I]iodoarylazidoprazoin for Pgp trapped in transition-state conformation (Pgp x ADP x vanadate) is reduced >30-fold. To transform Pgp from this intermediate state of low affinity for substrate to the next catalytic cycle, i.e., a conformation that binds substrate with high affinity, requires conditions that permit ATP hydrolysis. Additionally, there is an inverse correlation (R(2) = 0.96) between 8AzidoADP (or ADP) release and the recovery of substrate binding. These results suggest that the release of nucleotide is necessary for reactivation but not sufficient. The hydrolysis of additional molecule(s) of ATP (or 8AzidoATP) is obligatory for the catalytic cycle to advance to completion. These data are consistent with the observed stoichiometry of two ATP molecules hydrolyzed for the transport of every substrate molecule. Our data demonstrate two distinct roles for ATP hydrolysis in a single turnover of the catalytic cycle of Pgp, one in the transport of substrate and the other in effecting conformational changes to reset the pump for the next catalytic cycle.
Figures
Figure 1
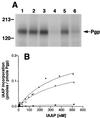
(A) Substrate binding to Pgp in Vi-induced ADP trapped conformation. Crude membranes (20 μg protein) were labeled with 5 nM IAAP after pretreatment with ATP or 8AzidoATP in the presence or absence of Vi as described in Materials and Methods. Autoradiogram shows untreated Pgp (lane 1); Pgp pretreated at 37°C for 10 min with, 1.25 mM ATP (lane 2); 250 μM Vi (lane 3); 1.25 mM ATP and 250 μM Vi (lane 4); 1.25 mM 8AzidoATP (lane 5), or 1.25 mM 8AzidoATP and 250 μM Vi (lane 6). (B) Saturation binding of IAAP to Pgp in normal and transition-state conformation. Crude membranes (20 μg protein) were labeled with increasing concentrations (0.7–516 nM) of IAAP after pretreatment with AMPPNP or ATP in the presence of 250 μM Vi. Samples were untreated (●), treated with AMPPNP (▴), or treated with ATP (■) as described in Materials and Methods and then labeled with IAAP. The data were fitted by using the software
graphpad prism
2.0 for the PowerPC MacIntosh and are representative of three independent experiments.
Figure 2
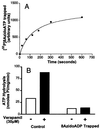
(A) Time course of Vi-induced trapping of 8Azido[α-32P]ADP on Pgp. Crude membranes (protein, 1 mg/ml) were incubated in the dark at 37°C with 50 μM 8Azido[α-32P]ATP (3–5 μCi/nmol) and 250 μM Vi in the ATPase assay buffer (see Materials and Methods). Aliquots were removed at indicated time points, reaction was terminated, and the samples were photocrosslinked by UV irradiation at 365 nm. After SDS/PAGE, radioactivity in the Pgp band was estimated by PhosphorImager analysis. (B) Basal and verapamil-stimulated ATP hydrolysis by Pgp before and after Vi trapping. ATPase activity of Pgp in crude membranes was measured by the endpoint Pi assay as described in Materials and Methods. Basal- (□) and verapamil- (30 μM) stimulated (■) ATPase was measured in control membranes and those that had been trapped into the Pgp⋅8AzidoADP⋅Vi complex, by incubation for 10 min with 1.25 mM 8AzidoATP and 250 μM Vi. Both control and Vi-trapped membranes were centrifuged to remove excess nucleotides and Vi before assaying the ATPase activity.
Figure 3
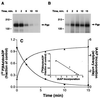
Relationship between the disassociation of 8AzidoADP and recovery of IAAP binding after Vi trapping. (A) Disassociation of 8Azido[α-32P]ADP from the Pgp⋅8Azido-[α-32P]ATP⋅Vi complex of Pgp. Crude membranes (protein, 1 mg/ml) were incubated in the dark at 37°C for 10 min with 50 μM 8Azido[α-32P]ATP (3–5 μCi/nmol) and 250 μM Vi in the ATPase assay buffer. The reaction was stopped by adding 12.5 mM ice-cold ATP and placing the tubes on ice. Untrapped nucleotides and excess Vi were removed by centrifugation at 300,000 × g for 10 min and the membranes were resuspended in the ATPase assay buffer. The resuspended membranes were placed at 37°C. Aliquots were removed at indicated intervals and placed on ice and photocrosslinked by UV irradiation at 365 nm for 5 min. (B) Recovery of IAAP binding to Pgp subsequent to formation of transition-state conformation. Crude membranes (1 mg/ml protein) were treated with 1.25 mM 8AzidoATP and 250 μM Vi for 10 min at 37°C in the ATPase assay buffer. The samples were placed on ice, and untrapped nucleotides and excess Vi were removed by centrifugation at 300,000 × g for 10 min at 4°C. The pellet was resuspended in the ATPase assay buffer containing 1.2 mM ATP and incubated at 37°C. Aliquots were transferred to ice at the indicated intervals and photolabeled with IAAP and visualized as described in Materials and Methods. Autoradiogram is shown in both A and B. (C) Relationship between the disassociation of 8AzidoADP and the recovery of IAAP binding after Vi trapping. The radioactivity associated with the Pgp bands depicted in A and B was quantified by using the Storm 860 PhosphorImager system. Disassociation of 8AzidoADP (●) and the increase in IAAP binding (▴) after Vi trapping were plotted as a function of time. (Inset) The increase in IAAP labeling, on the _x_-axis vs. the disassociation of 8Azido[α-32P]ADP on the _y_-axis, these are inversely proportional to each other with _R_2 = 0.959. The data are representative of three independent experiments.
Figure 4
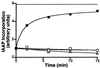
Recovery of IAAP binding to the Pgp⋅8AzidoADP⋅Vi complex requires ATP hydrolysis. Crude membranes (1 mg/ml protein) were treated with 1.25 mM 8AzidoATP and 250 μM Vi for 10 min at 37°C in the ATPase assay buffer (see Materials and Methods). The reaction was stopped by placing the samples on ice. Excess 8AzidoATP and Vi were removed by centrifugation at 300,000 × g for 10 min at 4°C. The pellet was resuspended in 40 mM Mes⋅Tris, pH6.8, 50 mM KCl, 5 mM sodium azide, 2 mM EGTA, and 2 mM DTT and divided into five aliquots to which the following additions were made: 10 mM MgCl2 and 5 mM AMPPNP (▵), 10 mM MgCl2, and 1.2 mM ATP (■), 5 mM EDTA (□), and 250 μM Vi (○). The continuous horizontal line shows the extent of IAAP incorporated into an equivalent amount of untreated control membranes, which were processed in parallel. The samples were incubated at 37°C, and aliquots were removed at the indicated intervals, placed on ice, and photocrosslinked with IAAP. After SDS/PAGE, the radioactivity associated with the Pgp was estimated by PhosphorImager analyses and normalized such that the radioactivity in the untreated sample at 0 min was 1. The data are representative of three independent experiments.
Figure 5
Effect of cyclosporin A and cis(Z)-flupentixol on the IAAP labeling of Pgp in the Vi-trapped and native conformations. (A) The effect of cyclosporin A and cis(Z)-flupentixol on the IAAP labeling of control and Pgp treated with 1.2 mM 8AzidoATP and 250 μM Vi at 37°C for 10 min immediately after trapping. After incubation at 37°C, the samples were centrifuged at 300,000 × g for 10 min at 4°C and resuspended in ice-cold ATPase assay buffer. The samples then were immediately incubated with cyclosporin A (1 μM) or cis(Z)-flupentixol (25 μM) for 3 min at room temperature, with IAAP (5 nM) for an additional 3 min, and then photocrosslinked as described in Materials and Methods. (B) Same experimental conditions as A except that the samples were incubated with 1.2 mM ATP for 15 min at 37°C before treatment with cyclosporin A or cis(Z)-flupentixol and labeling with IAAP. The various treatments are given above each autoradiogram.
Figure 6
A proposed scheme for the catalytic cycle of Pgp. The rectangles represent the N and C halves of Pgp and the overlapping circles the two NBS. The ovals represent the substrate binding sites, the on site is depicted by the continuous perimeter, and the off site with a dotted line (see Discussion for an explanation of the on and off sites). The hexagon portrays the on site with reduced affinity for the drug. Step I: Substrate binds to the on site of Pgp, and ATP binds to one or both of the two NBS. Step II: ATP is hydrolyzed and the drug is possibly moved to the lower affinity off site. Step III: Pi is released and the drug extruded from Pgp at this step. Step IIIA: When Pi is replaced by Vi, the Pgp⋅ADP⋅Vi complex is generated, which also exhibits a reduced affinity for substrate. Step IV/step IIIB: The ADP is disassociated; however, the affinity for substrate continues to be low. Step V: After disassociation of the ADP, an additional molecule(s) of ATP is hydrolyzed and the conformation of Pgp is restored to its original state with high affinity for substrate binding. Step VI: The release of Pi, disassociation of ADP followed by the initiation of the next catalytic cycle. It is not known, however, whether release of ADP necessarily precedes the binding of the next molecule of substrate after step VI. ADP disassociation (from the previous cycle), drug binding, and ATP binding presumably could occur in any sequence in steps VI to I. Though, we depict ATP binding and hydrolysis as occurring in the N half of Pgp our data do not suggest any preference in the presence of saturating concentrations of ATP or 8AzidoATP for either of the two sites, and the process is most likely random. However consistent with the alternate site model (4), ATP hydrolysis would occur at a site different from that in step II and therefore is depicted at the NBS in the C half at step V.
Similar articles
- Allosteric modulation bypasses the requirement for ATP hydrolysis in regenerating low affinity transition state conformation of human P-glycoprotein.
Maki N, Moitra K, Ghosh P, Dey S. Maki N, et al. J Biol Chem. 2006 Apr 21;281(16):10769-77. doi: 10.1074/jbc.M512579200. Epub 2006 Feb 27. J Biol Chem. 2006. PMID: 16505485 - The mechanism of action of multidrug-resistance-linked P-glycoprotein.
Sauna ZE, Smith MM, Müller M, Kerr KM, Ambudkar SV. Sauna ZE, et al. J Bioenerg Biomembr. 2001 Dec;33(6):481-91. doi: 10.1023/a:1012875105006. J Bioenerg Biomembr. 2001. PMID: 11804190 Review. - About a switch: how P-glycoprotein (ABCB1) harnesses the energy of ATP binding and hydrolysis to do mechanical work.
Sauna ZE, Ambudkar SV. Sauna ZE, et al. Mol Cancer Ther. 2007 Jan;6(1):13-23. doi: 10.1158/1535-7163.MCT-06-0155. Mol Cancer Ther. 2007. PMID: 17237262 Review.
Cited by
- Novel Potent ABCB1 Modulator, Phenethylisoquinoline Alkaloid, Reverses Multidrug Resistance in Cancer Cell.
Sugisawa N, Ohnuma S, Ueda H, Murakami M, Sugiyama K, Ohsawa K, Kano K, Tokuyama H, Doi T, Aoki J, Ishida M, Kudoh K, Naitoh T, Ambudkar SV, Unno M. Sugisawa N, et al. Mol Pharm. 2018 Sep 4;15(9):4021-4030. doi: 10.1021/acs.molpharmaceut.8b00457. Epub 2018 Aug 13. Mol Pharm. 2018. PMID: 30052463 Free PMC article. - Molecular basis of the polyspecificity of P-glycoprotein (ABCB1): recent biochemical and structural studies.
Chufan EE, Sim HM, Ambudkar SV. Chufan EE, et al. Adv Cancer Res. 2015;125:71-96. doi: 10.1016/bs.acr.2014.10.003. Epub 2015 Jan 8. Adv Cancer Res. 2015. PMID: 25640267 Free PMC article. Review. - PD173074, a selective FGFR inhibitor, reverses ABCB1-mediated drug resistance in cancer cells.
Patel A, Tiwari AK, Chufan EE, Sodani K, Anreddy N, Singh S, Ambudkar SV, Stephani R, Chen ZS. Patel A, et al. Cancer Chemother Pharmacol. 2013 Jul;72(1):189-99. doi: 10.1007/s00280-013-2184-z. Epub 2013 May 15. Cancer Chemother Pharmacol. 2013. PMID: 23673445 Free PMC article. - The FLT3 and PDGFR inhibitor crenolanib is a substrate of the multidrug resistance protein ABCB1 but does not inhibit transport function at pharmacologically relevant concentrations.
Mathias TJ, Natarajan K, Shukla S, Doshi KA, Singh ZN, Ambudkar SV, Baer MR. Mathias TJ, et al. Invest New Drugs. 2015 Apr;33(2):300-9. doi: 10.1007/s10637-015-0205-y. Epub 2015 Jan 20. Invest New Drugs. 2015. PMID: 25597754 Free PMC article. - The multidrug transporter Pdr5 on the 25th anniversary of its discovery: an important model for the study of asymmetric ABC transporters.
Golin J, Ambudkar SV. Golin J, et al. Biochem J. 2015 May 1;467(3):353-63. doi: 10.1042/BJ20150042. Biochem J. 2015. PMID: 25886173 Free PMC article. Review.
References
- Gottesman M M, Pastan I. Annu Rev Biochem. 1993;62:385–427. - PubMed
- Gottesman M M, Pastan I, Ambudkar S V. Curr Opin Genet Dev. 1996;6:610–617. - PubMed
- Ambudkar S V, Dey S, Hrycyna C A, Ramachandra M, Pastan I, Gottesman M M. Annu Rev Pharmacol Toxicol. 1999;39:361–398. - PubMed
- Senior A E, Al-Shawi M K, Urbatsch I L. FEBS Lett. 1995;377:285–289. - PubMed
- Ambudkar S V. Methods Enzymol. 1998;292:504–514. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous