Kainate receptor-mediated presynaptic inhibition at the mouse hippocampal mossy fibre synapse - PubMed (original) (raw)
Kainate receptor-mediated presynaptic inhibition at the mouse hippocampal mossy fibre synapse
H Kamiya et al. J Physiol. 2000.
Abstract
1. The presynaptic action of kainate (KA) receptor activation at the mossy fibre-CA3 synapse was examined using fluorescence measurement of presynaptic Ca2+ influx as well as electrophysiological recordings in mouse hippocampal slices. 2. Bath application of a low concentration (0.2 microM) of KA reversibly increased the amplitude of presynaptic volley evoked by stimulation of mossy fibres to 146 +/- 6 % of control (n = 6), whereas it reduced the field excitatory postsynaptic potential (EPSPs) to 30 +/- 4 %. 3. The potentiating effect of KA on the presynaptic volleys was also observed in Ca2+-free solution, and was partly antagonized by (2S, 4R)-4-methylglutamic acid (SYM 2081, 1 microM), which selectively desensitizes KA receptors. 4. The antidromic population spike of dentate granule cells evoked by stimulation of mossy fibres was increased by application of 0.2 microM KA to 160 +/- 10 % of control (n = 6). Whole-cell current-clamp recordings revealed that the stimulus threshold for generating antidromic spikes recorded from a single granule cell was lowered by KA application. 5. Application of KA (0.2 microM) suppressed presynaptic Ca2+ influx to 78 +/- 4 % of control (n = 6), whereas the amplitude of the presynaptic volley was increased. 6. KA at 0.2 microM reversibly suppressed excitatory postsynaptic currents (EPSCs) evoked by mossy fibre simulation to 38 +/- 9 % of control (n = 5). 7. These results suggest that KA receptor activation enhances the excitability of mossy fibres, probably via axonal depolarization, and reduces action potential-induced Ca2+ influx, thereby inhibiting mossy fibre EPSCs presynaptically. This novel presynaptic inhibitory action of KA at the mossy fibre-CA3 synapse may regulate the excitability of highly interconnected CA3 networks.
Figures
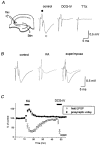
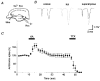
Figure 1. KA-induced enhancement of presynaptic volleys at the mossy fibre-CA3 synapse
A, schematic diagram showing experimental arrangement. A stimulating electrode (Stim) was placed on the stratum granulosum of the dentate gyrus, and the field potentials were recorded (Rec, recording electrode) from the stratum lucidum of the CA3 region. Traces are representative records of the field potentials recorded before and during application of 1 μM DCG-IV, a group II-selective metabotropic glutamate receptor (mGluR) agonist, and 0.5 μM tetrodotoxin (TTX). Note that the initial biphasic component (•, presynaptic volley) was not affected by DCG-IV, whereas the following negative component (^, field EPSP) was completely abolished. B, effects of KA (0.2 μM) on field potentials evoked by mossy fibre stimulation. Traces are representative field potentials recorded under the control conditions (left, continuous trace) and during application of KA (centre, dotted trace). The two traces are superimposed for comparison in the right panel. Note that the presynaptic volley potential was enhanced, whereas the field EPSP was depressed by KA application. C, the relative amplitudes of the presynaptic volley (•) and field EPSP (^), with those before KA application as references, are plotted against time (means ±
s.e.m.
, n = 6). KA (0.2 μM) and DCG-IV (1 μM) were applied during the period indicated.
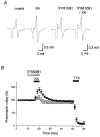
Figure 2. Effect of KA on the presynaptic volley potentials recorded in the Ca2+-free solution
A, representative traces of presynaptic volleys recorded in the Ca2+-free solution before and during KA application, either in the absence or presence of SYM 2081 which selectively desensitizes KA receptors. B, the time course of the presynaptic volley amplitude when KA (0.2 μM) and TTX (0.5 μM) were applied during the periods indicated (means ±
s.e.m.
, n = 6) is shown (•). In another set of experiments, 1 μM SYM 2081 was applied (^) during the period indicated by the open bar (n = 6).
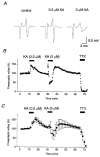
Figure 3. Effects of a higher concentration of KA
A, representative traces of presynaptic volleys recorded before and during application of KA (0.2 μM or 3 μM). B, the graph shows the time course of a single representative experiment. KA (0.2 μM or 3 μM) and TTX (0.5 μM) were applied during the periods indicated. Note that 3 μM KA briefly potentiated (arrow) but soon depressed presynaptic volley potentials during KA application. C, the graph shows the averaged time course of four experiments (means ±
s.e.m.
). The brief increase is not evident in the average because of variations in its time of occurrence.
Figure 4. Effect of KA on presynaptic volley evoked by stimulation of the mossy fibre axons
A, the stimulating electrode was placed on the stratum lucidum of the CA3c (hilar) region, to stimulate the mossy fibre pathway at the axon level. B, representative traces recorded before and during KA application. C, the graph shows the time course of the effect of KA on the amplitude of the presynaptic volley (means ±
s.e.m.
, n = 4). KA (0.2 μM) and TTX (0.5 μM) were applied during the periods indicated.
Figure 5. Effect of KA on antidromic population spikes evoked by mossy fibre stimulation
A, an electrical stimulus was delivered to the stratum lucidum of the CA3 region, and the evoked antidromic population spikes were recorded from the stratum granulosum of the dentate gyrus. B, specimen records of population spikes recorded before and during KA application. C, the graph represents the time course of the KA effect (means ±
s.e.m.
, n = 6). KA (0.2 μM) and TTX (0.5 μM) were applied during the periods indicated.
Figure 6. Effect of KA on antidromic spikes recorded from a single granule cell
A, antidromic spikes recorded using the whole-cell current-clamp method. Ten consecutive traces are superimposed for each condition. When weak stimulus (stim) intensity that failed to evoke antidromic spikes in some trials was used, KA application increased the probability of evoking antidromic spikes. The graph shows changes in the failure ratio before, during, and after KA application (n = 6). The stimulus intensity was set to evoke antidromic spikes in 10–90 % of all trials under control conditions. B, when the stimulus intensity was set strong enough to evoke antidromic spikes in all trials, KA (0.2 μM) caused a small depolarization and the amplitude of the antidromic spikes was slightly reduced (n = 4). C, effect of KA on membrane potentials (_V_m) of granule cells. _V_m was recorded continuously for 15 min, and 0.2 μM KA was applied during the period indicated by the open bar (5 min). Antidromic spikes were evoked every 30 s. Note that there were no inter-stimulus spontaneous antidromic spikes, which would be expected to occur if KA-induced axonal depolarization caused ectopic spikes at mossy fibre axons, during KA application (n = 5).
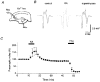
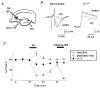
Figure 7. Suppression of presynaptic Ca2+ influx into mossy fibre terminals by KA
A, schematic diagram showing experimental arrangement. Membrane-permeable Ca2+ indicator rhod-2 AM was pressure-ejected into the stratum lucidum, resulting in selective loading of the presynaptic terminals through the mossy fibre pathway. Fluorescence from the labelled region, which had a diameter of about 100 μm and was about 500 μm away from the ejection site, was measured with a single photodiode. B, representative records of presynaptic Ca2+ transients (Δ_F/F_) and field EPSPs observed before (smooth traces) and during application of 0.2 μM KA (dotted traces). C, time courses of the effects of KA on presynaptic Ca2+ transient, presynaptic volley and field EPSP. The relative amplitudes of presynaptic Ca2+ transients (Δ_F/F_, •), presynaptic volley (▵) and field EPSP (^) evoked every 5 min were plotted as a function of time (means ±
s.e.m.
, n = 6). KA (0.2 μM) and a mixture of the AMPA receptor antagonist CNQX (10 μM) and the NMDA receptor antagonist D-AP5 (25 μM) were applied during the periods indicated.
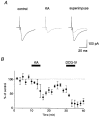
Figure 8. Effect of KA on mossy fibre EPSCs
A, representative EPSCs recorded in the control solution and during application of 0.2 μM KA. B, time course of the effect of KA on the EPSCs (means ±
s.e.m.
, n = 5). KA (0.2 μM) and DCG-IV (1 μM) were applied during the period as indicated. These EPSCs were abolished almost completely by addition of the group II-selective mGluR agonist DCG-IV (1 μM).
Similar articles
- Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus.
Kamiya H, Ozawa S. Kamiya H, et al. J Physiol. 1998 Jun 15;509 ( Pt 3)(Pt 3):833-45. doi: 10.1111/j.1469-7793.1998.833bm.x. J Physiol. 1998. PMID: 9596803 Free PMC article. - Dual mechanism for presynaptic modulation by axonal metabotropic glutamate receptor at the mouse mossy fibre-CA3 synapse.
Kamiya H, Ozawa S. Kamiya H, et al. J Physiol. 1999 Jul 15;518 ( Pt 2)(Pt 2):497-506. doi: 10.1111/j.1469-7793.1999.0497p.x. J Physiol. 1999. PMID: 10381595 Free PMC article. - Calcium-dependent mechanisms involved in presynaptic long-term depression at the hippocampal mossy fibre-CA3 synapse.
Kobayashi K, Manabe T, Takahashi T. Kobayashi K, et al. Eur J Neurosci. 1999 May;11(5):1633-8. doi: 10.1046/j.1460-9568.1999.00578.x. Eur J Neurosci. 1999. PMID: 10215916 - Kainate receptors and the induction of mossy fibre long-term potentiation.
Bortolotto ZA, Lauri S, Isaac JT, Collingridge GL. Bortolotto ZA, et al. Philos Trans R Soc Lond B Biol Sci. 2003 Apr 29;358(1432):657-66. doi: 10.1098/rstb.2002.1216. Philos Trans R Soc Lond B Biol Sci. 2003. PMID: 12740111 Free PMC article. Review. - Presynaptic kainate receptors at hippocampal mossy fiber synapses.
Schmitz D, Mellor J, Frerking M, Nicoll RA. Schmitz D, et al. Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11003-8. doi: 10.1073/pnas.191351498. Proc Natl Acad Sci U S A. 2001. PMID: 11572960 Free PMC article. Review.
Cited by
- Kainate receptor signaling in pain pathways.
Bhangoo SK, Swanson GT. Bhangoo SK, et al. Mol Pharmacol. 2013 Feb;83(2):307-15. doi: 10.1124/mol.112.081398. Epub 2012 Oct 24. Mol Pharmacol. 2013. PMID: 23095167 Free PMC article. Review. - Metabotropic regulation of intrinsic excitability by synaptic activation of kainate receptors.
Melyan Z, Lancaster B, Wheal HV. Melyan Z, et al. J Neurosci. 2004 May 12;24(19):4530-4. doi: 10.1523/JNEUROSCI.5356-03.2004. J Neurosci. 2004. PMID: 15140923 Free PMC article. - Timing and efficacy of Ca2+ channel activation in hippocampal mossy fiber boutons.
Bischofberger J, Geiger JR, Jonas P. Bischofberger J, et al. J Neurosci. 2002 Dec 15;22(24):10593-602. doi: 10.1523/JNEUROSCI.22-24-10593.2002. J Neurosci. 2002. PMID: 12486151 Free PMC article. - Neto1 is an auxiliary subunit of native synaptic kainate receptors.
Tang M, Pelkey KA, Ng D, Ivakine E, McBain CJ, Salter MW, McInnes RR. Tang M, et al. J Neurosci. 2011 Jul 6;31(27):10009-18. doi: 10.1523/JNEUROSCI.6617-10.2011. J Neurosci. 2011. PMID: 21734292 Free PMC article. - Domoic acid-induced neurotoxicity in the hippocampus of adult rats.
Chandrasekaran A, Ponnambalam G, Kaur C. Chandrasekaran A, et al. Neurotox Res. 2004;6(2):105-17. doi: 10.1007/BF03033213. Neurotox Res. 2004. PMID: 15325963 Review.
References
- Bettler B, Mulle C. Neurotransmitter receptors II. AMPA and kainate receptors. Neuropharmacology. 1995;34:123–139. - PubMed
- Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. - PubMed
- Cattaert D, El-Manira A, Clarac F. Chloride conductance produces both presynaptic inhibition and antidromic spikes in primary afferents. Brain Research. 1994;666:109–112. - PubMed
- Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous