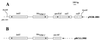Characterization of VIM-2, a carbapenem-hydrolyzing metallo-beta-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France - PubMed (original) (raw)
Characterization of VIM-2, a carbapenem-hydrolyzing metallo-beta-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France
L Poirel et al. Antimicrob Agents Chemother. 2000 Apr.
Abstract
Pseudomonas aeruginosa COL-1 was identified in a blood culture of a 39-year-old-woman treated with imipenem in Marseilles, France, in 1996. This strain was resistant to beta-lactams, including ureidopenicillins, ticarcillin-clavulanic acid, cefepime, ceftazidime, imipenem, and meropenem, but remained susceptible to the monobactam aztreonam. The carbapenem-hydrolyzing beta-lactamase gene of P. aeruginosa COL-1 was cloned, sequenced, and expressed in Escherichia coli DH10B. The deduced 266-amino-acid protein was an Ambler class B beta-lactamase, with amino acid identities of 32% with B-II from Bacillus cereus; 31% with IMP-1 from several gram-negative rods in Japan, including P. aeruginosa; 27% with CcrA from Bacteroides fragilis; 24% with BlaB from Chryseobacterium meningosepticum; 24% with IND-1 from Chryseobacterium indologenes; 21% with CphA-1 from Aeromonas hydrophila; and 11% with L-1 from Stenotrophomonas maltophilia. It was most closely related to VIM-1 beta-lactamase recently reported from Italian P. aeruginosa clinical isolates (90% amino acid identity). Purified VIM-2 beta-lactamase had a pI of 5.6, a relative molecular mass of 29.7 kDa, and a broad substrate hydrolysis range, including penicillins, cephalosporins, cephamycins, oxacephamycins, and carbapenems, but not monobactams. As a metallo-beta-lactamase, its activity was zinc dependent and inhibited by EDTA (50% inhibitory concentration, 50 microM). VIM-2 conferred a resistance pattern to beta-lactams in E. coli DH10B that paralleled its in vitro hydrolytic properties, except for susceptibility to ureidopenicillins, carbapenems, and cefepime. bla(VIM-2) was located on a ca. 45-kb plasmid that in addition conferred resistance to sulfamides and that was not self-transmissible either from P. aeruginosa to E. coli or from E. coli to E. coli. bla(VIM-2) was the only gene cassette located within the variable region of a novel class 1 integron, In56, that was weakly related to the bla(VIM-1)-containing integron. VIM-2 is the second carbapenem-hydrolyzing metalloenzyme characterized from a P. aeruginosa isolate outside Japan.
Figures
FIG. 1
(A) Schematic map of the recombinant plasmid pNOR-2001 encoding _bla_VIM-2 (arrows indicate its translational orientation) and (B) comparison with the _bla_VIM-1-containing integron as cloned into recombinant plasmid pBCLL/39H (10). For pNOR-2001, the solid line represents the cloned insert from P. aeruginosa COL-1 with the ORFs that are boxed, and the dotted lines indicate the vector pBK-CMV.
FIG. 2
Nucleotide sequence of the 3,843-bp fragment of the cloned _Bam_HI-fragment of pNOR-2001 containing the _bla_VIM-2 coding region and its integron. The deduced amino acid sequence is designated in the single-letter code below the nucleotide sequence. The start codons of IntI1, _bla_VIM-2, qacEΔ1, and sul1 genes are indicated by horizontal arrows, and their stop codons are indicated by asterisks. Only the start and the end of the integrase, qacEΔ1, sul1, and orf5 genes are represented. The −35 and −10 sequences of the promoters Pc, P2, and Pint are underlined; RBS indicates the putative ribosome binding site for _bla_VIM-2. The conserved core and inverse core sites located at the _bla_VIM-2 cassette boundaries are boxed, and the composite 59-base element is italicized. The cassette boundaries are indicated by vertical arrows. The left part of the attI1 site is underlined with a dotted line.
FIG. 3
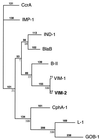
Dendrogram obtained for 10 representative Ambler class B carbapenem-hydrolyzing β-lactamases by parsimony analysis (16). The alignment used for tree calculation was performed with Clustal W (16) followed by minor adjustments in order to reduce the number of gaps and to maintain the alignment of the amino acid residues identified as critical for activity of some class B carbapenem-hydrolyzing β-lactamases. Branch lengths are drawn to scale and are proportional to the number of amino acid changes. The percentage values at branching points (underlined) refer to the number of times a particular node was found in 100 bootstrap replications (the star indicates uncertainty about nodes with bootstrap values of less than 50%). The distance along the vertical axis has no significance. The origins of the β-lactamases are given in Table 3.
FIG. 4
Comparison of the amino acid sequence of VIM-2 with that of VIM-1. Identical amino acid residues are indicated by asterisks, and functionally equivalent amino acid substitutions are indicated by colons. Boldface amino acids are those of the putative active sites of VIM-1 and of VIM-2. The numbering is according to the B-II sequence of B. cereus (18).
FIG. 5
Comparison of the sequences of the 72 bp of the _bla_VIM-2 59-base element (59-be) and the 81 bp of the _bla_VIM-1 59-base element present in the circular form of the β-lactamase gene cassettes to a 59-base element consensus sequence given below. The inverse core and core sequences are double underlined. L1, L2, R1, and R2 are four regions found to be highly conserved within 59-base elements of class 1 integrons (20, 23). Consensus bases (Cons) in uppercase letters are present in two-thirds or more of the 59-base elements, and bases in lowercase letters are present in half or more of the 59-base elements. Stars indicate bases of the 59-base element of _bla_VIM-2 that fit the consensus. R, purine; Y, pyrimidine; S, C or G; N, undetermined base.
Similar articles
- Cloning and characterization of blaVIM, a new integron-borne metallo-beta-lactamase gene from a Pseudomonas aeruginosa clinical isolate.
Lauretti L, Riccio ML, Mazzariol A, Cornaglia G, Amicosante G, Fontana R, Rossolini GM. Lauretti L, et al. Antimicrob Agents Chemother. 1999 Jul;43(7):1584-90. doi: 10.1128/AAC.43.7.1584. Antimicrob Agents Chemother. 1999. PMID: 10390207 Free PMC article. - Characterization of the new metallo-beta-lactamase VIM-13 and its integron-borne gene from a Pseudomonas aeruginosa clinical isolate in Spain.
Juan C, Beceiro A, Gutiérrez O, Albertí S, Garau M, Pérez JL, Bou G, Oliver A. Juan C, et al. Antimicrob Agents Chemother. 2008 Oct;52(10):3589-96. doi: 10.1128/AAC.00465-08. Epub 2008 Jul 21. Antimicrob Agents Chemother. 2008. PMID: 18644957 Free PMC article. - Analysis of a novel class 1 integron containing metallo-beta-lactamase gene VIM-2 in Pseudomonas aeruginosa.
Jeong JH, Shin KS, Lee JW, Park EJ, Son SY. Jeong JH, et al. J Microbiol. 2009 Dec;47(6):753-9. doi: 10.1007/s12275-008-0272-2. Epub 2010 Feb 4. J Microbiol. 2009. PMID: 20127470 - Acquired carbapenem-hydrolyzing beta-lactamases and their genetic support.
Poirel L, Nordmann P. Poirel L, et al. Curr Pharm Biotechnol. 2002 Jun;3(2):117-27. doi: 10.2174/1389201023378427. Curr Pharm Biotechnol. 2002. PMID: 12022255 Review. - Molecular epidemiology of carbapenem-resistant Pseudomonas aeruginosa in an endemic area: comparison with global data.
Karampatakis T, Antachopoulos C, Tsakris A, Roilides E. Karampatakis T, et al. Eur J Clin Microbiol Infect Dis. 2018 Jul;37(7):1211-1220. doi: 10.1007/s10096-018-3244-4. Epub 2018 Apr 11. Eur J Clin Microbiol Infect Dis. 2018. PMID: 29644540 Review.
Cited by
- Structural basis for carbapenem-hydrolyzing mechanisms of carbapenemases conferring antibiotic resistance.
Jeon JH, Lee JH, Lee JJ, Park KS, Karim AM, Lee CR, Jeong BC, Lee SH. Jeon JH, et al. Int J Mol Sci. 2015 Apr 29;16(5):9654-92. doi: 10.3390/ijms16059654. Int J Mol Sci. 2015. PMID: 25938965 Free PMC article. Review. - Evaluation of a new Etest for detecting metallo-beta-lactamases in routine clinical testing.
Walsh TR, Bolmström A, Qwärnström A, Gales A. Walsh TR, et al. J Clin Microbiol. 2002 Aug;40(8):2755-9. doi: 10.1128/JCM.40.8.2755-2759.2002. J Clin Microbiol. 2002. PMID: 12149325 Free PMC article. - Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-beta-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp.
Lee K, Lim YS, Yong D, Yum JH, Chong Y. Lee K, et al. J Clin Microbiol. 2003 Oct;41(10):4623-9. doi: 10.1128/JCM.41.10.4623-4629.2003. J Clin Microbiol. 2003. PMID: 14532193 Free PMC article. - Ertapenem resistance of Escherichia coli.
Lartigue MF, Poirel L, Poyart C, Réglier-Poupet H, Nordmann P. Lartigue MF, et al. Emerg Infect Dis. 2007 Feb;13(2):315-7. doi: 10.3201/eid1302.060747. Emerg Infect Dis. 2007. PMID: 17479901 Free PMC article. - First outbreak of a plasmid-mediated carbapenem-hydrolyzing OXA-48 beta-lactamase in Klebsiella pneumoniae in Spain.
Pitart C, Solé M, Roca I, Fàbrega A, Vila J, Marco F. Pitart C, et al. Antimicrob Agents Chemother. 2011 Sep;55(9):4398-401. doi: 10.1128/AAC.00329-11. Epub 2011 Jul 11. Antimicrob Agents Chemother. 2011. PMID: 21746954 Free PMC article.
References
- Bellais S, Léotard S, Poirel L, Naas T, Nordmann P. Molecular characterization of a carbapenem-hydrolyzing β-lactamase from Chryseobacterium (Flavobacterium) indologenes. FEMS Microbiol Lett. 1999;171:127–132. - PubMed
- Bush K. Metallo-enzymes: a class apart. Clin Infect Dis. 1998;27(Suppl. 1):S48–S53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous