Induction of apoptotic cell death in peripheral blood mononuclear and polymorphonuclear cells by an oral bacterium, Fusobacterium nucleatum - PubMed (original) (raw)
Induction of apoptotic cell death in peripheral blood mononuclear and polymorphonuclear cells by an oral bacterium, Fusobacterium nucleatum
A Jewett et al. Infect Immun. 2000 Apr.
Abstract
It is largely unknown why a variety of bacteria present in the oral cavity are capable of establishing themselves in the periodontal pockets of nonimmunocompromised individuals in the presence of competent immune effector cells. In this paper we present evidence for the immunosuppressive role of Fusobacterium nucleatum, a gram-negative oral bacterium which plays an important role in the generation of periodontal disease. Our studies indicate that the immunosuppressive role of F. nucleatum is largely due to the ability of this organism to induce apoptotic cell death in peripheral blood mononuclear cells (PBMCs) and in polymorphonuclear cells (PMNs). F. nucleatum treatment induced apoptosis of PBMCs and PMNs as assessed by an increase in subdiploid DNA content determined by DNA fragmentation and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling assays. The ability of F. nucleatum to induce apoptosis was abolished by either heat treatment or proteinase digestion but was retained after formaldehyde treatment, suggesting that a heat-labile surface protein component is responsible for bacterium-mediated cell apoptosis. The data also indicated that F. nucleatum-induced cell apoptosis requires activation of caspases and is protected by NF-kappaB. Possible mechanisms of F. nucleatum's role in the pathogenesis of periodontal disease are discussed.
Figures
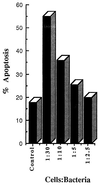
FIG. 1
Dose-dependent induction of apoptotic cell death of PBMCs by F. nucleatum. PBMCs were cocultured in the presence of F. nucleatum at the indicated ratios. The levels of apoptotic cell death were determined by using flow cytometric analysis of propidium iodide-stained cells.
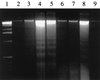
FIG. 2
Inhibition of _F. nucleatum_-mediated apoptotic cell death of PBMCs by the ICE inhibitor. Jurkat cells were cocultured in the presence of F. nucleatum and P. intermedia (30:1 bacterium-cell ratio) overnight. Equal amounts of DNA (2 μg) extracted from equal numbers of cells for each sample were loaded onto a 2% agarose gel and run on a gel electrophoresis assay. Lanes: 1, molecular weight marker; 2, untreated Jurkat cells; 3, Jurkat cells treated with viable P. intermedia; 4, Jurkat cells treated with viable F. nucleatum; 5, Jurkat cells treated with 1% formaldehyde-treated F. nucleatum; 6, Jurkat cells treated with viable F. nucleatum and 500 μM ICE inhibitor YVAD; 7, Jurkat cells treated with 1% formaldehyde-treated F. nucleatum and YVAD; 8, Jurkat cells treated with anti-FAS antibody; 9, Jurkat cells treated with Anti-FAS antibody and YVAD.
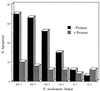
FIG. 3
Inhibition of _F. nucleatum_-induced apoptotic cell death of PBMCs by pronase. F. nucleatum was cultured for 18 h in the presence or absence of pronase (10 mg/ml) (protease type XIV; EC 3.4.24.31) prior to its addition to Jurkat cells. The Jurkat cells were then cocultured for 18 h with either the pronase-treated F. nucleatum (+ Pronase) or control untreated F. nucleatum (− Pronase). Jurkat cell apoptosis was determined by flow cytometric analysis of propidium iodide-stained cells.
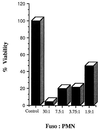
FIG. 4
Induction of cell death in peripheral blood PMNs by F. nucleatum. PMNs at a concentration of 2 × 106 per ml were cocultured in the presence of F. nucleatum for 16 h. The numbers of viable cells were counted by trypan blue staining.
Similar articles
- The role of aggregation in Fusobacterium nucleatum- induced immune cell death.
Huynh T, Kapur RV, Kaplan CW, Cacalano N, Kinder Haake S, Shi W, Sieling P, Jewett A. Huynh T, et al. J Endod. 2011 Nov;37(11):1531-5. doi: 10.1016/j.joen.2011.06.034. Epub 2011 Sep 3. J Endod. 2011. PMID: 22000457 - Fusobacterium nucleatum T18 aggregates human mononuclear cells and inhibits their PHA-stimulated proliferation.
Kinder Haake S, Lindemann RA. Kinder Haake S, et al. J Periodontol. 1997 Jan;68(1):39-44. doi: 10.1902/jop.1997.68.1.39. J Periodontol. 1997. PMID: 9029450 - Strain-Specific Impact of Fusobacterium nucleatum on Neutrophil Function.
Kurgan Ş, Kansal S, Nguyen D, Stephens D, Koroneos Y, Hasturk H, Van Dyke TE, Kantarci A. Kurgan Ş, et al. J Periodontol. 2017 Apr;88(4):380-389. doi: 10.1902/jop.2016.160212. Epub 2016 Oct 20. J Periodontol. 2017. PMID: 27762731 - The role of Fusobacterium nucleatum in cancer and its implications for clinical applications.
Luo W, Han J, Peng X, Zhou X, Gong T, Zheng X. Luo W, et al. Mol Oral Microbiol. 2024 Dec;39(6):417-432. doi: 10.1111/omi.12475. Epub 2024 Jul 11. Mol Oral Microbiol. 2024. PMID: 38988217 Review. - Fusobacterium nucleatum and oral cancer: a critical review.
McIlvanna E, Linden GJ, Craig SG, Lundy FT, James JA. McIlvanna E, et al. BMC Cancer. 2021 Nov 13;21(1):1212. doi: 10.1186/s12885-021-08903-4. BMC Cancer. 2021. PMID: 34774023 Free PMC article. Review.
Cited by
- Trichomonas vaginalis promotes apoptosis of human neutrophils by activating caspase-3 and reducing Mcl-1 expression.
Kang JH, Song HO, Ryu JS, Shin MH, Kim JM, Cho YS, Alderete JF, Ahn MH, Min DY. Kang JH, et al. Parasite Immunol. 2006 Sep;28(9):439-46. doi: 10.1111/j.1365-3024.2006.00884.x. Parasite Immunol. 2006. PMID: 16916367 Free PMC article. - Periodontal pathogens and cancer development.
Zhou Y, Meyle J, Groeger S. Zhou Y, et al. Periodontol 2000. 2024 Oct;96(1):112-149. doi: 10.1111/prd.12590. Epub 2024 Jul 4. Periodontol 2000. 2024. PMID: 38965193 Free PMC article. Review. - Oral commensal bacteria differentially modulate epithelial cell death.
White T, Alimova Y, Alves VTE, Emecen-Huja P, Al-Sabbagh M, Villasante A, Ebersole JL, Gonzalez OA. White T, et al. Arch Oral Biol. 2020 Dec;120:104926. doi: 10.1016/j.archoralbio.2020.104926. Epub 2020 Oct 7. Arch Oral Biol. 2020. PMID: 33096404 Free PMC article. - Identification and characterization of a novel adhesin unique to oral fusobacteria.
Han YW, Ikegami A, Rajanna C, Kawsar HI, Zhou Y, Li M, Sojar HT, Genco RJ, Kuramitsu HK, Deng CX. Han YW, et al. J Bacteriol. 2005 Aug;187(15):5330-40. doi: 10.1128/JB.187.15.5330-5340.2005. J Bacteriol. 2005. PMID: 16030227 Free PMC article. - Dying for a cause: The pathogenic manipulation of cell death and efferocytic pathways.
Cooper KN, Potempa J, Bagaitkar J. Cooper KN, et al. Mol Oral Microbiol. 2024 Aug;39(4):165-179. doi: 10.1111/omi.12436. Epub 2023 Oct 2. Mol Oral Microbiol. 2024. PMID: 37786286 Review.
References
- Ali R W, Skaug N, Nilsen R, Bakken V. Microbial associations of 4 putative periodontal pathogens in Sudanese adult periodontitis patients determined by DNA probe analysis. J Periodontal. 1994;65:1053–1057. - PubMed
- Ali R W, Bakken V, Nilsen R, Skaug N. Comparative detection frequency of 6 putative periodontal pathogens in Sudanese and Norwegian adult periodontitis patients. J Periodontal. 1994;65:1046–1052. - PubMed
- Baeuerle P A, Baltimore D. NF-κB: ten years later. Cell. 1996;87:13–20. - PubMed
- Baichwal V R, Baeuerle P A. Apoptosis: activate NF-κB or die? Curr Biol. 1997;7:R94–R96. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources