Determination of cell adhesion sites of neuropilin-1 - PubMed (original) (raw)
Determination of cell adhesion sites of neuropilin-1
M Shimizu et al. J Cell Biol. 2000.
Abstract
Neuropilin-1 is a type 1 membrane protein with three distinct functions. First, it can mediate cell adhesion via a heterophilic molecular interaction. Second, in neuronal cells, neuropilin-1 binds the class 3 semaphorins, which are neuronal chemorepellents, and plays a role in the directional guidance of axons. Neuropilin-1 is expected to form complexes with the plexinA subfamily members and mediate the semaphorin-elicited inhibitory signals into neurons. Third, in endothelial cells, neuropilin-1 binds a potent endothelial cell mitogen, vascular endothelial growth factor (VEGF)(165), and regulates vessel formation. Though the binding sites in neuropilin-1 for the class 3 semaphorins and VEGF(165) have been analyzed, the sites involved in cell adhesion activity of the molecule have not been identified. In this study, we produced a variety of mutant neuropilin-1s and tested their cell adhesion activity. We showed that the b1 and b2 domains within the extracellular segment of neuropilin-1 were required for the cell adhesion activity, and peptides with an 18-amino acid stretch in the b1 and b2 domains were sufficient to induce the cell adhesion activity. In addition, we demonstrated that the cell adhesion ligands for neuropilin-1 were proteins and distributed in embryonic mesenchymal cells but distinct from the class 3 semaphorins, VEGF, or plexins.
Figures
Figure 1
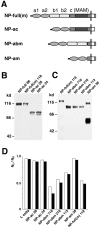
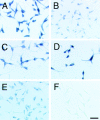
Cell adhesion activity of transfectants which express mutant neuropilin-1 proteins (Part 1). (A) A schematic representation of mutant neuropilin-1s. NP-full, intact neuropilin-1; NP-bc, neuropilin-1 lacking the a1 and a2 domains; NP-c, neuropilin-1 protein lacking the a1, a2, b1, and b2 domains; NP-abcp, neuropilin-1 whose transmembrane and cytoplasmic regions are replaced by that of the Xenopus plexin. (B) Immunoblot of transfectants expressing intact and mutant neuropilin-1 proteins, by using an antibody raised against the b1-c domains. The number followed by the name of the construct represents the clone number. (C) Quantification of cell aggregation activity of the mutant neuropilin-1s. The degree of aggregation of transfectants is expressed by the index Nt/N0, where Nt and N0 are the total particle number at incubation times t and 0, respectively. White and black bars represent N30/N0 and N60/N0, respectively. (D–F) Cell aggregation at 60 min in gyration, detected by phase contrast (D and E) or fluorescence microscope (F). Parental L cells do not show cell aggregability (D). In contrast, fluorescein-labeled parental L cells and transfectants expressing NP-full 86 form mixed cell aggregates (E and F). E and F show the same field. Bar, 100 μm.
Figure 2
Cell adhesion activity of transfectants which express mutant neuropilin-1 proteins (Part 2). (A) A schematic representation of mutant neuropilin-1s; NP-full(m), full-length neuropilin-1 containing myc tag at the COOH-terminal end; NP-ac, neuropilin-1 lacking the b1 and b2 domains; NP-abm, neuropilin-1 lacking the c domain (myc-tagged); NP-am, neuropilin-1 lacking the b1, b2, and c domains (myc-tagged). (B and C) Immunoblot of the transfectants with the antibody raised against the b1-c domains (B) and anti-myc antibody (C). The number followed by the name of the construct represents the clone number. A band at the 100-kD position in the lane of NP-am appears to be dimerized proteins. (D) Quantification of cell aggregation activity of the mutant neuropilin-1s. White and black bars represent N30/N0 and N60/N0, respectively.
Figure 3
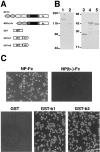
Cell adhesion activity of the recombinant neuropilin-1 proteins. (A) A schematic representation of the Fc-tagged full-length neuropilin-1 extracellular segment (NP-Fc) and the b1-b2 domains–deleted one [NP(b-)-Fc], and GST-tagged recombinant b1 (GST-b1) and b2 (GST-b2) domains. (B) SDS-PAGE of the recombinant proteins stained with Coomassie brilliant blue G-250. Lanes 1–5 correspond to NP-Fc, NP(b-)-Fc, GST, GST-b1, and GST-b2 recombinant proteins, respectively. (C) Cell adhesion to the recombinant proteins immobilized on nitrocellulose-coated culture dishes. L cells adhere to Fc-NP, GST-b1, and GST-b2 but not NP(b-)-Fc and GST. Bar, 100 μm.
Figure 4
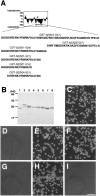
Determination of cell adhesion sites in the b2 domain. (A) A list of synthesized peptides in the b2 domain. The upper figure represents the Kyte-Doolittle hydoropathy plot of the b2 domain. An asterisk indicates a putative glycosylation site. An arrow indicates the region to which a series of GST-tagged recombinant proteins are synthesized. The number in the name of each recombinant protein indicates the position of the amino acid residues. (B) SDS-PAGE of the affinity-purified recombinant proteins. Lane 1, GST; lane 2, GST-b2(501–547); lane 3, GST-b2(525–547); lane 4, GST-b2(501–524); lane 5, GST-b2(501–521); lane 6, GST-b2(501–516); lane 7, GST-b2(504–521); and lane 8, GST-b1(347–364; see the legend for Fig. 5). The gel was stained with Coomassie brilliant blue G-250. (C–I) Adhesion of L cells to the immobilized recombinant proteins at 30 min. C, GST-b2(501–547); D, GST-b2(525–547); E, GST-b2(501–524); F, GST-b2(501–521); G, GST-b2(501–516); H, GST-b2(504–521). Trypsin-treated L cells did not adhere to GST-b2(504–521) (I). Bar, 100 μm.
Figure 5
Determination of cell adhesion sites in the b1 domain. (A) Amino acid alignment of the b1 and b2 domains. Shaded boxes indicate consensus sequences. An open box indicates the cell adhesion sites in the b2 domain and its homologous region (347–364 aa) in the b1 domain. (B and C) Cell adhesion activity of the GST-tagged b1(347–364) recombinant protein [GST-b1(347–364)]. L cells (B) but not trypsin-treated L cells (C) adhere to the immobilized GST-b1(347–364). Bar, 100 μm.
Figure 6
Adhesion of embryonic mesenchymal cells to the recombinant proteins for the cell adhesion sites of neuropilin-1. Trunk mesenchymal cells from E13 mouse embryos adhere to the immobilized GST-b1(347–364) (A and B) and GST-b2(504–521) (C and D) recombinant proteins. Trypsin-treated mesenchymal cells do not adhere to the recombinant proteins (B and D). Bar, 100 μm.
Figure 8
Binding of semaphorins, VEGF, and plexins to the recombinant neuropilin-1 proteins. (A) Bindings of SEMA3A-AP, Sema3B-AP and Sema3C-AP to the immobilized NP-Fc, GST, GST-b1, GST-b2, GST-b1(347–364), and GST-b2(504–521) were visualized with NBT/BCIP. (B and C) Adhesion of L cells to the immobilized NP-Fc in the presence of SEMA3A (C) and VEGF165 (D). SEMA3A and VEGF165 do not interfere with the binding of L cells to NP-Fc. (D and E) Binding of GST-tagged recombinant proteins for the cell adhesion sites of neuropilin-1 to myc-tagged plexinA3 expressed in COS-7 cells. The plexinA3 was visualized by immunohistochemistry with anti-myc antibody (D). GST-b1(347–364) and GST-b2(504–521) bound to the cells were detected by immunohistochemistry with anti-GST antibody. Bars, 100 μm.
Figure 7
Binding of AP-tagged semaphorin 3A (SEMA3A-AP) to mutant neuropilin-1 proteins. Binding of SEMA3A-AP to the transfectants expressing NP-full 86 (A; see Fig. 1 B), NP-bc 30 (B; see Fig. 1 B), NP-ac 24 (C; see Fig. 2 B), NP-abm 716 (D; see Fig. 2 C), NP-am 38 (E; see Fig. 2 C), and NP-c 68 (F; see Fig. 1 B). SEMA3A-AP bound to cell surface was visualized with NBT/BCIP. Bar, 100 μm.
Figure 9
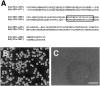
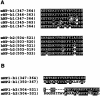
Comparison of amino acid sequences in cell adhesion sites among vertebrate neuropilin-1s (A) or the mouse neuropilin family (B). The amino acid sequences in the cell adhesion sites are highly conserved among the mouse (mNP), rat (rNP), human (hNP), chicken (cNP), and Xenopus (xNP) neuropilin-1s. On the other hand, only about half of the amino acid residues of the cell adhesion site of mouse neuropilin-1 and of mouse neuropilin-2 are identical.
Similar articles
- Characterization of neuropilin-1 structural features that confer binding to semaphorin 3A and vascular endothelial growth factor 165.
Gu C, Limberg BJ, Whitaker GB, Perman B, Leahy DJ, Rosenbaum JS, Ginty DD, Kolodkin AL. Gu C, et al. J Biol Chem. 2002 May 17;277(20):18069-76. doi: 10.1074/jbc.M201681200. Epub 2002 Mar 8. J Biol Chem. 2002. PMID: 11886873 - Vascular endothelial growth factor (VEGF)-like protein from orf virus NZ2 binds to VEGFR2 and neuropilin-1.
Wise LM, Veikkola T, Mercer AA, Savory LJ, Fleming SB, Caesar C, Vitali A, Makinen T, Alitalo K, Stacker SA. Wise LM, et al. Proc Natl Acad Sci U S A. 1999 Mar 16;96(6):3071-6. doi: 10.1073/pnas.96.6.3071. Proc Natl Acad Sci U S A. 1999. PMID: 10077638 Free PMC article. - Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity.
Gagnon ML, Bielenberg DR, Gechtman Z, Miao HQ, Takashima S, Soker S, Klagsbrun M. Gagnon ML, et al. Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2573-8. doi: 10.1073/pnas.040337597. Proc Natl Acad Sci U S A. 2000. PMID: 10688880 Free PMC article. - Neuropilin is a mediator of angiogenesis.
Miao HQ, Klagsbrun M. Miao HQ, et al. Cancer Metastasis Rev. 2000;19(1-2):29-37. doi: 10.1023/a:1026579711033. Cancer Metastasis Rev. 2000. PMID: 11191060 Review. - Roles of a neuronal cell-surface molecule, neuropilin, in nerve fiber fasciculation and guidance.
Fujisawa H, Kitsukawa T, Kawakami A, Takagi S, Shimizu M, Hirata T. Fujisawa H, et al. Cell Tissue Res. 1997 Nov;290(2):465-70. doi: 10.1007/s004410050954. Cell Tissue Res. 1997. PMID: 9321711 Review.
Cited by
- Oligo-guanosine nucleotide induces neuropilin-1 internalization in endothelial cells and inhibits angiogenesis.
Narazaki M, Segarra M, Hou X, Tanaka T, Li X, Tosato G. Narazaki M, et al. Blood. 2010 Oct 21;116(16):3099-107. doi: 10.1182/blood-2010-01-265801. Epub 2010 Jul 6. Blood. 2010. PMID: 20606164 Free PMC article. - Molecular dynamics simulations, docking and MMGBSA studies of newly designed peptide-conjugated glucosyloxy stilbene derivatives with tumor cell receptors.
Rico MI, Lebedenko CG, Mitchell SM, Banerjee IA. Rico MI, et al. Mol Divers. 2022 Oct;26(5):2717-2743. doi: 10.1007/s11030-021-10354-9. Epub 2022 Jan 17. Mol Divers. 2022. PMID: 35037187 - Proteome dynamics during postnatal mouse corpus callosum development.
Son AI, Fu X, Suto F, Liu JS, Hashimoto-Torii K, Torii M. Son AI, et al. Sci Rep. 2017 Mar 28;7:45359. doi: 10.1038/srep45359. Sci Rep. 2017. PMID: 28349996 Free PMC article. - Semaphorin3D regulates axon axon interactions by modulating levels of L1 cell adhesion molecule.
Wolman MA, Regnery AM, Becker T, Becker CG, Halloran MC. Wolman MA, et al. J Neurosci. 2007 Sep 5;27(36):9653-63. doi: 10.1523/JNEUROSCI.1741-07.2007. J Neurosci. 2007. PMID: 17804626 Free PMC article. - Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function.
Williams CK, Li JL, Murga M, Harris AL, Tosato G. Williams CK, et al. Blood. 2006 Feb 1;107(3):931-9. doi: 10.1182/blood-2005-03-1000. Epub 2005 Oct 11. Blood. 2006. PMID: 16219802 Free PMC article.
References
- Beckmann G., Bork P. An adhesive domain detected in functionally diverse receptors. Trends Biochem. Sci. 1993;18:40–41. - PubMed
- Chen H., Chédotal A., He Z., Goodman C.S., Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. - PubMed
- Cheng H.J., Flanagan J.G. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994;79:157–168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases