Vaccination with tat toxoid attenuates disease in simian/HIV-challenged macaques - PubMed (original) (raw)
Vaccination with tat toxoid attenuates disease in simian/HIV-challenged macaques
C D Pauza et al. Proc Natl Acad Sci U S A. 2000.
Abstract
The Tat protein is essential for HIV type 1 (HIV-1) replication and may be an important virulence factor in vivo. We studied the role of Tat in viral pathogenesis by immunizing rhesus macaques with chemically inactivated Tat toxoid and challenging these animals by intrarectal inoculation with the simian/human immunodeficiency virus 89.6PD. Immune animals had significantly attenuated disease with lowered viral RNA, interferon-alpha, and chemokine receptor expression (CXCR4 and CCR5) on CD4(+) T cells; these features of infection have been linked to in vitro effects of Tat and respond similarly to extracellular Tat protein produced during infection. Immunization with Tat toxoid inhibits key steps in viral pathogenesis and should be included in therapeutic or preventive HIV-1 vaccines.
Figures
Figure 1
Immune responses and virus burden at set-point (56 days after inoculation) for immunized and control animals challenged by intrarectal inoculation with SHIV89.6PD. Samples collected before challenge were characterized for anti-Tat serum antibody titers (reciprocal of greatest dilution that was positive by ELISA) and Tat-specific lymphoproliferative responses (stimulation indices). Data were separated according to the plasma viral burden at 56 days after infection (set-point); A shows low viral burden (open symbols), and B (closed symbols) shows high viral burden. Symbols indicate whether animals were immunized with Tat toxoid (circle), Tat toxoid plus gp160 (square), Tat (triangle), or Group B control (asterisk). Data points are labeled with the animal identification numbers.
Figure 2
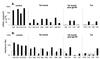
Immunization decreases plasma virus burden and IFN-α accumulation. The upper four panels (A) show plasma viral RNA levels at 4 weeks after SHIV89.6PD infection in immunized or control macaques. Data are expressed on a semilogarithmic scale with a lower limit of sensitivity at 2,500 copies per ml. The lower four panels (B) show levels of plasma IFN-α on the day of challenge (open bars) and 8 weeks after challenge (solid bars). IFN-α levels were measured as described in the text. Results are expressed as reciprocal of the largest plasma dilution that produced a 50% reduction in the plaque count. Individual data point are coded with the last three digits of each animal identification number.
Figure 3
Changes in CD4 T cell count for control and immunized animals through 8 weeks after virus challenge. CD4+ T cell counts were obtained from flow cytometry analysis (to determine CD4 T cell percentage) and the absolute lymphocyte count in blood (from automated cell counts). Data are provided for individual macaques (identified in each legend).
Figure 4
Levels of chemokine receptor expression on circulating CD4+ T cells at 28 days after virus inoculation in immunized and control macaques. The ordinate shows the percentage of CD4+ T cells that were positive for expression of cell surface CCR5 or CXCR4. The lower levels of CCR5 expression were statistically significant for all immunized groups compared with control (P ≤ 0.002), and the lower levels of CXCR4 were statistically significant for immunized groups compared with control (P ≤ 0.02).
Similar articles
- SHIV89.6P pathogenicity in cynomolgus monkeys and control of viral replication and disease onset by human immunodeficiency virus type 1 Tat vaccine.
Cafaro A, Caputo A, Maggiorella MT, Baroncelli S, Fracasso C, Pace M, Borsetti A, Sernicola L, Negri DR, Ten Haaft P, Betti M, Michelini Z, Macchia I, Fanales-Belasio E, Belli R, Corrias F, Buttò S, Verani P, Titti F, Ensoli B. Cafaro A, et al. J Med Primatol. 2000 Aug;29(3-4):193-208. doi: 10.1034/j.1600-0684.2000.290313.x. J Med Primatol. 2000. PMID: 11085582 - Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs.
Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Harouse JM, et al. Science. 1999 Apr 30;284(5415):816-9. doi: 10.1126/science.284.5415.816. Science. 1999. PMID: 10221916 - Evaluation in rhesus macaques of Tat and rev-targeted immunization as a preventive vaccine against mucosal challenge with SHIV-BX08.
Verrier B, Le Grand R, Ataman-Onal Y, Terrat C, Guillon C, Durand PY, Hurtrel B, Aubertin AM, Sutter G, Erfle V, Girard M. Verrier B, et al. DNA Cell Biol. 2002 Sep;21(9):653-8. doi: 10.1089/104454902760330183. DNA Cell Biol. 2002. PMID: 12396607 - Activated CD4+CCR5+ T cells in the rectum predict increased SIV acquisition in SIVGag/Tat-vaccinated rhesus macaques.
Carnathan DG, Wetzel KS, Yu J, Lee ST, Johnson BA, Paiardini M, Yan J, Morrow MP, Sardesai NY, Weiner DB, Ertl HC, Silvestri G. Carnathan DG, et al. Proc Natl Acad Sci U S A. 2015 Jan 13;112(2):518-23. doi: 10.1073/pnas.1407466112. Epub 2014 Dec 30. Proc Natl Acad Sci U S A. 2015. PMID: 25550504 Free PMC article.
Cited by
- Sterilizing Immunity against COVID-19: Developing Helper T cells I and II activating vaccines is imperative.
Kyei-Barffour I, Addo SA, Aninagyei E, Ghartey-Kwansah G, Acheampong DO. Kyei-Barffour I, et al. Biomed Pharmacother. 2021 Dec;144:112282. doi: 10.1016/j.biopha.2021.112282. Epub 2021 Oct 2. Biomed Pharmacother. 2021. PMID: 34624675 Free PMC article. Review. - Polyphosphazene immunoadjuvants: Historical perspective and recent advances.
Andrianov AK, Langer R. Andrianov AK, et al. J Control Release. 2021 Jan 10;329:299-315. doi: 10.1016/j.jconrel.2020.12.001. Epub 2020 Dec 5. J Control Release. 2021. PMID: 33285104 Free PMC article. Review. - Tat-Based Therapies as an Adjuvant for an HIV-1 Functional Cure.
Jin H, Li D, Lin MH, Li L, Harrich D. Jin H, et al. Viruses. 2020 Apr 8;12(4):415. doi: 10.3390/v12040415. Viruses. 2020. PMID: 32276443 Free PMC article. Review. - Anti-Tat Immunity in HIV-1 Infection: Effects of Naturally Occurring and Vaccine-Induced Antibodies Against Tat on the Course of the Disease.
Cafaro A, Tripiciano A, Picconi O, Sgadari C, Moretti S, Buttò S, Monini P, Ensoli B. Cafaro A, et al. Vaccines (Basel). 2019 Aug 26;7(3):99. doi: 10.3390/vaccines7030099. Vaccines (Basel). 2019. PMID: 31454973 Free PMC article. Review. - The grafting of universal T-helper epitopes enhances immunogenicity of HIV-1 Tat concurrently improving its safety profile.
Kashi VP, Jacob RA, Shamanna RA, Menon M, Balasiddaiah A, Varghese RK, Bachu M, Ranga U. Kashi VP, et al. PLoS One. 2014 Dec 22;9(12):e114155. doi: 10.1371/journal.pone.0114155. eCollection 2014. PLoS One. 2014. PMID: 25531437 Free PMC article.
References
- Cullen B. J Virol. 1990;63:655–657. - PubMed
- Ensoli B, Barillari G, Salahuddin S Z, Gallo R C, Wong-Staal F. Nature (London) 1990;345:84–86. - PubMed
- Frankel A D, Pabo C O. Cell. 1988;55:1189–1193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials