Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus - PubMed (original) (raw)
Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus
A F Hoffman et al. J Neurosci. 2000.
Abstract
The localization of cannabinoid (CB) receptors to GABAergic interneurons in the hippocampus indicates that CBs may modulate GABAergic function and thereby mediate some of the disruptive effects of marijuana on spatial memory and sensory processing. To investigate the possible mechanisms through which CB receptors may modulate GABAergic neurotransmission in the hippocampus, whole-cell voltage-clamp recordings were performed on CA1 pyramidal neurons in rat brain slices. Stimulus-evoked GABA(A) receptor-mediated IPSCs were reduced in a concentration-dependent manner by the CB receptor agonist WIN 55,212-2 (EC(50) of 138 nM). This effect was blocked by the CB1 receptor antagonist SR141716A (1 microM) but not by the opioid antagonist naloxone. In contrast, evoked GABA(B)-mediated IPSCs were insensitive to the CB agonist. WIN 55,212-2 also reduced the frequency of spontaneous, action potential-dependent IPSCs (sIPSCs), without altering action potential-independent miniature IPSCs (mIPSCs), measured while sodium channels were blocked by tetrodotoxin (TTX). Blockade of voltage-dependent calcium channels (VDCCs) by cadmium also eliminated the effect of WIN 55,212-2 on sIPSCs. Depolarization of inhibitory terminals with elevated extracellular potassium caused a large increase in the frequency of mIPSCs that was inhibited by both cadmium and WIN 55,212-2. The presynaptic effect of WIN 55,212-2 was also investigated using the potassium channel blockers barium and 4-aminopyridine. Neither of these agents significantly altered the effect of WIN 55,212-2 on evoked IPSCs. Together, these data suggest that presynaptic CB1 receptors reduce GABA(A)- but not GABA(B)-mediated synaptic inhibition of CA1 pyramidal neurons by inhibiting VDCCs located on inhibitory nerve terminals.
Figures
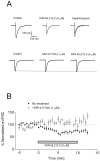
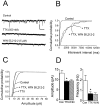
Fig. 1.
Effect of WIN 55,212–2 on stimulus-evoked GABAA receptor-mediated IPSCs in CA1 pyramidal neurons. Whole-cell recordings were performed using CsCl-based electrode solution at a holding potential of −80 mV. Current_traces_ represent an average of 5–10 sweeps.A, In the top series of_traces_, application of 5 μ
m
WIN 55,212–2 reduced the GABAA IPSC. Control and drug_traces_ are superimposed for clarity. In a different cell shown in the bottom series of traces, application of SR141716A (1 μ
m
; 10 min) did not alter the IPSC. In the continued presence of SR141716A, WIN 55,212–2 had no effect on the IPSC. B, Summary of the effect of WIN 55–212, plotted as a percentage change (mean ± SEM) from control. Closed circles represent the effect of WIN 55,212–2 alone (n = 12), and open circles represent the effect of WIN 55,212–2 after pretreatment with SR141716A (n = 9).
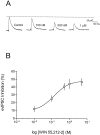
Fig. 2.
Concentration-dependent effect of WIN 55,212–2 on evoked GABAA IPSCs in CA1 pyramidal neurons.A, Recording from a single neuron using a K+-gluconate-based electrode solution at a holding potential of −55 mV. Control and WIN 55,212–2 concentrations are labeled for each trace. Traces represents an average of 5–10 sweeps taken at the peak of a stable drug response.B, Concentration–response curve for WIN 55,212–2. Each data point represents the mean ± SEM of the maximal inhibition of the evoked IPSC (n = 3–12 cells). The EC50 estimated from the fitted curve is 138 n
m
.
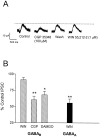
Fig. 3.
Effect of WIN 55,212–2 on GABABreceptor-mediated IPSCs. A, Recording from a single neuron clamped at −55 mV using K+-gluconate-based internal solution. APV (40 μ
m
), DNQX (10 μ
m
), and picrotoxin (100 μ
m
) were included in the superfusion medium. Traces represent an average of 8–10 sweeps. Application of the GABAB antagonist CGP 35348 (100 μ
m
) reduced the IPSC amplitude, and this effect reversed within ∼10 min. Subsequent application of WIN 55,212–2 (1 μ
m
; 15 min) did not decrease the IPSC.B, Summary of the effects of WIN 55,212–2 (1 μ
m
; n = 7) (WIN), CGP 35348 (100 μ
m
;n = 6) (CGP), and the μ-opioid agonist DAMGO (1 μ
m
; n = 4) on the GABAB response (*p < 0.05, **p < 0.01 vs control; one-way ANOVA, followed by Tukey–Kramer post hoc analysis). For comparison, the effect of 1 μ
m
WIN 55,212–2 on the GABAAreceptor-mediated IPSC is shown (solid bar) (n = 6; **p < 0.01 vs WIN effect on GABAB IPSC; unpaired Student's _t_test).
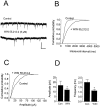
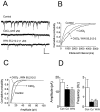
Fig. 4.
Effect of WIN 55,212–2 on spontaneous, action potential-dependent IPSCs in a CA1 pyramidal neuron. Recording was performed using a CsCl-based electrode solution at a holding potential of −80 mV. A, Traces represent portions of 2 min epochs recorded before (Control) and during the peak drug effect (∼7 min after 5 μ
m
WIN 55,212–2 application). B, Cumulative interevent interval distribution shown for the same cell, revealing a significant increase in the interevent interval (i.e., decreased frequency;p < 0.001; K–S test) during WIN 55,212–2 application. C, Cumulative amplitude distribution obtained from the same cell reveals a significant decrease in sIPSC amplitude (p < 0.001; K–S test) in the presence of WIN 55,212–2. The mean sIPSC amplitude in this cell was decreased from −29.5 pA (n = 392 events) to −18.4 pA (n = 145 events). D, Summary of the effect of 5 μ
m
WIN 55,212–2 (WIN) on the amplitude and frequency of sIPSCs (mean ± SEM; n = 5). Significant reductions were observed in both amplitude and frequency (*p< 0.05; Wilcoxon signed rank test). Calibration: 50 pA, 500 msec.
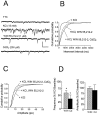
Fig. 5.
Effect of WIN 55,212–2 on action potential-independent (TTX-insensitive) mIPSCs in a single CA1 pyramidal neuron. Holding potential, −80 mV. A,Traces represent portions of 2 min epochs recorded before TTX application (Control), 10 min into the TTX (500 n
m
) application, and 10 min into the WIN 55,212–2 (5 μ
m
) application. B, Cumulative interevent interval distributions for each treatment condition in the same cell. A significant decrease in the frequency of events was observed during TTX (p < 0.001; K–S test). During WIN 55,212–2 application, no further change (_p_ > 0.05; K–S test) in the distribution was observed. C, Cumulative amplitude distribution for the same cell demonstrating a decrease in amplitude during TTX (p < 0.001; K–S test). The mean amplitude decreased from −12.6 pA (n = 518 events) to −8.8 pA (n = 43 events) in TTX. However, no further change in the mean amplitude (−9.3 pA; n = 56 events) was observed during WIN 55,212–2 treatment. D, Summary of mIPSC amplitude and frequency (mean ± SEM;n = 6) before TTX (Con, open bars), during TTX (filled bars) (*p < 0.05 vs control; Wilcoxon signed rank test), and during WIN 55,212–2 (5 μ
m
) (hatched bars). Calibration: 25 pA, 500 msec.
Fig. 6.
Effect of WIN 55,212–2 on spontaneous IPSCs in a CA1 pyramidal neuron during CdCl2 application. Holding potential, −80 mV. A, Traces represent portions of 2 min epochs obtained before treatment (Control), 10 min into CdCl2 (200 μ
m
) application, and 13 min into WIN 55,212–2 (1 μ
m
) application. B, Cumulative interevent interval distribution for the same cell. CdCl2 produced a significant increase in the interevent interval (p < 0.001; K–S test). During WIN 55,212–2 treatment in the presence of CdCl2, there was no further change in the distribution (_p_> 0.05; K–S test). C, Cumulative amplitude distribution for the same cell reveals that CdCl2 treatment significantly (p < 0.001; K–S test) reduced the amplitude from control. The mean amplitude decreased from −17.9 pA (_n_ = 241 events) in control to −13.0 pA (_n_ = 126 events) during CdCl2. A significant change in the amplitude distribution was not observed during WIN 55,212–2 application in the presence of CdCl2(_p_ > 0.05; K–S test; mean amplitude, −10.7 pA; n = 92 events). D, Summary of mean ± SEM amplitude and frequency changes of sIPSCs (n = 6) during CdCl2(Cd) (*p < 0.05 vs control; Wilcoxon signed rank test) and WIN 55,212–2 in the presence of CdCl2 (WIN). Calibration: 25 pA, 500 msec.
Fig. 7.
Effect of WIN 55,212–2 on mIPSCs during depolarization of inhibitory terminals with elevated [K+]o. Holding potential, −80 mV using a CsCl-based internal solution. A,Traces representing portions of 2 min epochs acquired during the sequential administration of TTX (500 n
m
; in standard 3 m
m
[K+]o), elevated [K+]o (KCl) (15 m
m
), WIN 55,212–2 (1 μ
m
), and CdCl2 (200 μ
m
). B, Cumulative interevent interval distribution for the same cell. WIN 55,212–2 produced a significant (p < 0.001; K–S test) increase in the interevent interval distribution during application of high [K+]o and TTX, indicating a decrease in frequency. Subsequent application of CdCl2 also resulted in a significant (p < 0.001; K–S test) shift in the interevent interval distribution, indicating that the direct activation of VDCCs contributed to the enhancement of mIPSC frequency in elevated [K+]o. C, Cumulative amplitude distribution for the same cell. Both WIN 55,212–2 and the subsequent application of CdCl2 produced a significant (p < 0.001; K–S test) decrease in the cumulative amplitude distribution relative to high [K+]o. The mean amplitudes were as follows: high [K+], −10.6 pA (n = 637 events); WIN 55,212–2, −8.4 pA (n = 367 events); and CdCl2, −7.9 pA (n = 264 events). D, Summary of the effects of WIN 55,212–2 (WIN) (1 μ
m
; n = 6) and CdCl2(Cd) (200 μ
m;
n = 6) on the mean frequency and amplitude. Data represent the mean ± SEM and are shown as a percentage of the baseline obtained in high [K+]o (*p < 0.05 vs baseline; Student's two-tailed t test). Although the mean amplitude was not significantly affected, a significant (p < 0.001; K–S test) shift in the cumulative amplitude distribution was observed in four of six cells. Calibration: 25 pA, 500 msec.
Fig. 8.
Effect of K+ channel blockade on WIN 55,212–2-mediated inhibition of evoked GABAA-mediated IPSCs in CA1 pyramidal neurons. Recordings were performed using a K+-gluconate-based electrode solution at a holding potential of −45 to −55 mV. A, Under standard conditions (2.4 m
m
CaCl2), BaCl2 (300 μ
m
) (top traces) and 4-AP (100 μ
m
) (bottom traces) produced a large increase in the IPSC. Application of WIN 55,212–2 (1 μ
m
) in the presence of BaCl2 or 4-AP failed to cause a significant reduction in the IPSC. B, Under conditions in which the extracellular Ca2+ was reduced to 1.2–2.0 m
m
after BaCl2 (top trace) or 4-AP (bottom trace) application, WIN 55,212–2 reduced the IPSC.C, Summary of the effects of WIN 55,212–2 (1 μ
m
) in the presence of BaCl2 (300 μ
m
) or 4-AP (100 μ
m
) under either standard (open bars) or low Ca2+(hatched bars) aCSF. Data represent the mean ± SEM of the number of cells given in parentheses. The horizontal dashed lines represent the effect of 1 μ
m
WIN 55,212–2 on GABAA IPSCs in standard aCSF without BaCl2 or 4-AP (mean ± SEM; n = 4).
Similar articles
- Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons.
Huang CC, Lo SW, Hsu KS. Huang CC, et al. J Physiol. 2001 May 1;532(Pt 3):731-48. doi: 10.1111/j.1469-7793.2001.0731e.x. J Physiol. 2001. PMID: 11313442 Free PMC article. - Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids.
Hoffman AF, Lupica CR. Hoffman AF, et al. J Neurophysiol. 2001 Jan;85(1):72-83. doi: 10.1152/jn.2001.85.1.72. J Neurophysiol. 2001. PMID: 11152707 - Cannabinoid receptor modulation of synapses received by cerebellar Purkinje cells.
Takahashi KA, Linden DJ. Takahashi KA, et al. J Neurophysiol. 2000 Mar;83(3):1167-80. doi: 10.1152/jn.2000.83.3.1167. J Neurophysiol. 2000. PMID: 10712447 - Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition.
Hájos N, Freund TF. Hájos N, et al. Chem Phys Lipids. 2002 Dec 31;121(1-2):73-82. doi: 10.1016/s0009-3084(02)00149-4. Chem Phys Lipids. 2002. PMID: 12505692 Review. - Cannabinoids, hippocampal function and memory.
Hampson RE, Deadwyler SA. Hampson RE, et al. Life Sci. 1999;65(6-7):715-23. doi: 10.1016/s0024-3205(99)00294-5. Life Sci. 1999. PMID: 10462072 Review.
Cited by
- Drug-Induced Alterations of Endocannabinoid-Mediated Plasticity in Brain Reward Regions.
Zlebnik NE, Cheer JF. Zlebnik NE, et al. J Neurosci. 2016 Oct 5;36(40):10230-10238. doi: 10.1523/JNEUROSCI.1712-16.2016. J Neurosci. 2016. PMID: 27707960 Free PMC article. Review. - Region-specific developmental specialization of GABA-glycine cosynapses in laminas I-II of the rat spinal dorsal horn.
Keller AF, Coull JA, Chery N, Poisbeau P, De Koninck Y. Keller AF, et al. J Neurosci. 2001 Oct 15;21(20):7871-80. doi: 10.1523/JNEUROSCI.21-20-07871.2001. J Neurosci. 2001. PMID: 11588160 Free PMC article. - Heterosynaptic GABAB Receptor Function within Feedforward Microcircuits Gates Glutamatergic Transmission in the Nucleus Accumbens Core.
Manz KM, Baxley AG, Zurawski Z, Hamm HE, Grueter BA. Manz KM, et al. J Neurosci. 2019 Nov 20;39(47):9277-9293. doi: 10.1523/JNEUROSCI.1395-19.2019. Epub 2019 Oct 2. J Neurosci. 2019. PMID: 31578230 Free PMC article. - Cannabinoids Occlude the HIV-1 Tat-Induced Decrease in GABAergic Neurotransmission in Prefrontal Cortex Slices.
Xu C, Hermes DJ, Mackie K, Lichtman AH, Ignatowska-Jankowska BM, Fitting S. Xu C, et al. J Neuroimmune Pharmacol. 2016 Jun;11(2):316-31. doi: 10.1007/s11481-016-9664-y. Epub 2016 Mar 18. J Neuroimmune Pharmacol. 2016. PMID: 26993829 Free PMC article. - Cannabinoids for the treatment of schizophrenia? A balanced neurochemical framework for both adverse and therapeutic effects of cannabis use.
Coulston CM, Perdices M, Henderson AF, Malhi GS. Coulston CM, et al. Schizophr Res Treatment. 2011;2011:501726. doi: 10.1155/2011/501726. Epub 2010 Jul 27. Schizophr Res Treatment. 2011. PMID: 22937266 Free PMC article.
References
- Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–348. - PubMed
- Axelrod J, Felder CC. Cannabinoid receptors and their endogenous agonist, anandamide. Neurochem Res. 1998;23:575–581. - PubMed
- Breivogel CS, Childers SR. The functional neuroanatomy of brain cannabinoid receptors. Neurobiol Dis. 1998;5:417–431. - PubMed
- Buhl EH, Cobb SR, Halasy K, Somogyi P. Properties of unitary IPSPs evoked by anatomically identified basket cells in the rat hippocampus. Eur J Neurosci. 1995;7:1989–2004. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous