NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area - PubMed (original) (raw)
NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area
C Racca et al. J Neurosci. 2000.
Abstract
Glutamate receptors activated by NMDA (NMDARs) or AMPA (AMPARs) are clustered on dendritic spines of pyramidal cells. Both the AMPAR-mediated postsynaptic responses and the synaptic AMPAR immunoreactivity show a large intersynapse variability. Postsynaptic responses mediated by NMDARs show less variability. To assess the variability in NMDAR content and the extent of their coexistence with AMPARs in Schaffer collateral-commissural synapses of adult rat CA1 pyramidal cells, electron microscopic immunogold localization of receptors has been used. Immunoreactivity of NMDARs was detected in virtually all synapses on spines, but AMPARs were undetectable, on average, in 12% of synapses. A proportion of synapses had a very high AMPAR content relative to the mean content, resulting in a distribution more skewed toward larger values than that of NMDARs. The variability of synaptic NMDAR content [coefficient of variation (CV), 0.64-0.70] was much lower than that of the AMPAR content (CV, 1.17-1.45). Unlike the AMPAR content, the NMDAR content showed only a weak correlation with synapse size. As reported previously for AMPARs, the immunoreactivity of NMDARs was also associated with the spine apparatus within spines. The results demonstrate that the majority of the synapses made by CA3 pyramidal cells onto spines of CA1 pyramids express both NMDARs and AMPARs, but with variable ratios. A less-variable NMDAR content is accompanied by a wide variability of AMPAR content, indicating that the regulation of expression of the two receptors is not closely linked. These findings support reports that fast excitatory transmission at some of these synapses is mediated by activation mainly of NMDARs.
Figures
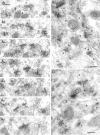
Fig. 1.
Variability in the NMDA receptor content of synapses on spines of CA1 pyramidal cells in the stratum radiatum.A1–A7, Electron micrographs of serial sections show the high probability of labeling synapses with a mixture of the antibodies ab-NR1-pan and ab-NR2A/B. All five synapses (1–5) are immunopositive. The spine bearing perforated synapse_number_ 2 (_2_′, 2", segments of postsynaptic density) contains a labeled spine apparatus (asterisk; A4, A5).B, C, In a single section of type I synapses made by boutons (b) with spines (s) and a dendritic shaft (d), only some synapses (large arrows) contain immunoparticles; others appear immunonegative (small arrows). The same bouton can form synapses with several spines (crossed arrows). Note that gold particles often cluster at the synapses. Scale bars, 0.2 μm.
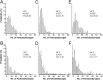
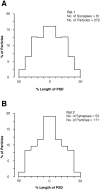
Fig. 2.
Distribution of synapses on spines according to NMDAR (A, C, E) and AMPAR (B, D, F) immunoreactivity in three adult rats (A, B, rat 1; C, D, rat 2; and E, F, rat 3). The distributions of synapses are skewed toward larger values for both receptors. Virtually all synapses contain NMDAR, but 14.4% (B), 12.8% (D), and 9.4% (F) of synapses are immunonegative for AMPA receptor. The distributions of AMPAR content are more skewed and have a larger coefficient of variation (CV). The sample presented in D contained three synapses having >60 particles for AMPAR. Data for B and D are from Nusser et al. (1998).
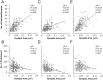
Fig. 3.
NMDAR immunoparticle density at synapses on spines. A, C, E, There is a weak positive correlation between the size and the NMDA receptor immunoparticle content of synapses (p < 0.01, Spearman rank correlation). A, For rat 1, slope of regression line = 155.4 gold/μm2. C, For rat 2, slope of regression line = 78.1 gold/μm2. E, For rat 3, slope of regression line = 153.0 gold/μm2.B, D, F, The gold particle density (number of particles per square micrometer) for NMDARs is weakly and negatively correlated with synapse size (Spearman rank correlation).
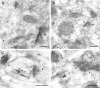
Fig. 4.
Double immunogold labeling of NMDARs (10 nm particles) and AMPARs (5 nm particles; arrows) in individual synapses on spines (s) in the stratum radiatum. b, Bouton. Scale bars: A, 0.1 μm; B–D, 0.2 μm.
Fig. 5.
Average tangential distribution of immunogold labeling of NMDARs (mixture of antibodies ab-NR1-pan and ab-NR2A/B) along the postsynaptic density of CA1 pyramidal cell spines in rats 1 (A) and 2 (B). The radial location of gold particles was measured from the midline of the PSD and normalized across the synapse population having variable size. The distribution obtained was mirrored across the midline for display. Labeling of NMDARs has a higher probability toward the center of the PSD.
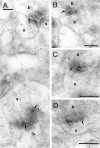
Fig. 6.
Immunoreactivity of NMDARs and AMPARs in the spine apparatus of hippocampal CA1 pyramidal cells. A, B, Gold particles for NMDAR are found in the synaptic junctions (thick arrows) and in the spine apparatus (asterisks) of spines. C, D, Double labeling of NMDARs (large particles, 10 nm in C and 20 nm in D) and AMPARs (small particles, 5 nm in_C_ and 10 nm in D; thin arrows) shows that both receptors are localized in the same spine apparatus. b, Bouton; d, dendritic shaft. Scale bars, 0.2 μm.
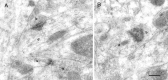
Fig. 7.
NMDAR-immunopositive synapses on dendritic shafts (d) in stratum radiatum of the CA1 area.A, B, Gold particles can also be observed within the dendrite in A. The synapse in B is cut tangential. b, Bouton; s, spine. Scale bar: A, B, 0.2 μm.
Similar articles
- Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities.
Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Ganeshina O, et al. J Comp Neurol. 2004 Jan 1;468(1):86-95. doi: 10.1002/cne.10950. J Comp Neurol. 2004. PMID: 14648692 - Large variability in synaptic N-methyl-D-aspartate receptor density on interneurons and a comparison with pyramidal-cell spines in the rat hippocampus.
Nyíri G, Stephenson FA, Freund TF, Somogyi P. Nyíri G, et al. Neuroscience. 2003;119(2):347-63. doi: 10.1016/s0306-4522(03)00157-x. Neuroscience. 2003. PMID: 12770551 - TARP γ-2 and γ-8 Differentially Control AMPAR Density Across Schaffer Collateral/Commissural Synapses in the Hippocampal CA1 Area.
Yamasaki M, Fukaya M, Yamazaki M, Azechi H, Natsume R, Abe M, Sakimura K, Watanabe M. Yamasaki M, et al. J Neurosci. 2016 Apr 13;36(15):4296-312. doi: 10.1523/JNEUROSCI.4178-15.2016. J Neurosci. 2016. PMID: 27076426 Free PMC article. - Making of a Synapse: Recurrent Roles of Drebrin A at Excitatory Synapses Throughout Life.
Aoki C, Sherpa AD. Aoki C, et al. Adv Exp Med Biol. 2017;1006:119-139. doi: 10.1007/978-4-431-56550-5_8. Adv Exp Med Biol. 2017. PMID: 28865018 Review. - Regulation of AMPA receptor recruitment at developing synapses.
Hall BJ, Ghosh A. Hall BJ, et al. Trends Neurosci. 2008 Feb;31(2):82-9. doi: 10.1016/j.tins.2007.11.010. Epub 2008 Jan 16. Trends Neurosci. 2008. PMID: 18201773 Review.
Cited by
- Contribution of cytoskeleton to the internalization of AMPA receptors.
Zhou Q, Xiao M, Nicoll RA. Zhou Q, et al. Proc Natl Acad Sci U S A. 2001 Jan 30;98(3):1261-6. doi: 10.1073/pnas.98.3.1261. Epub 2001 Jan 23. Proc Natl Acad Sci U S A. 2001. PMID: 11158627 Free PMC article. - Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity.
Deller T, Korte M, Chabanis S, Drakew A, Schwegler H, Stefani GG, Zuniga A, Schwarz K, Bonhoeffer T, Zeller R, Frotscher M, Mundel P. Deller T, et al. Proc Natl Acad Sci U S A. 2003 Sep 2;100(18):10494-9. doi: 10.1073/pnas.1832384100. Epub 2003 Aug 19. Proc Natl Acad Sci U S A. 2003. PMID: 12928494 Free PMC article. - Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD.
Petersen JD, Chen X, Vinade L, Dosemeci A, Lisman JE, Reese TS. Petersen JD, et al. J Neurosci. 2003 Dec 3;23(35):11270-8. doi: 10.1523/JNEUROSCI.23-35-11270.2003. J Neurosci. 2003. PMID: 14657186 Free PMC article. - From the stochasticity of molecular processes to the variability of synaptic transmission.
Ribrault C, Sekimoto K, Triller A. Ribrault C, et al. Nat Rev Neurosci. 2011 Jun 20;12(7):375-87. doi: 10.1038/nrn3025. Nat Rev Neurosci. 2011. PMID: 21685931 Review. - The substrates of memory: defects, treatments, and enhancement.
Lynch G, Rex CS, Chen LY, Gall CM. Lynch G, et al. Eur J Pharmacol. 2008 May 6;585(1):2-13. doi: 10.1016/j.ejphar.2007.11.082. Epub 2008 Mar 4. Eur J Pharmacol. 2008. PMID: 18374328 Free PMC article. Review.
References
- Baude A, Nusser Z, Roberts JDB, Mulvihill E, McIlhinney RAJ, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. - PubMed
- Bernard V, Bolam JP. Subcellular and subsynaptic distribution of the NR1 subunit of the NMDA receptor in the neostriatum and globus pallidus of the rat: co-localization at synapses with the GluR2/3 subunit of the AMPA receptor. Eur J Neurosci. 1998;10:3721–3736. - PubMed
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. - PubMed
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous