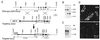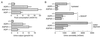Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels - PubMed (original) (raw)
Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels
T Ma et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Aquaporin-3 (AQP3) is a water channel expressed at the basolateral plasma membrane of kidney collecting-duct epithelial cells. The mouse AQP3 cDNA was isolated and encodes a 292-amino acid water/glycerol-transporting glycoprotein expressed in kidney, large airways, eye, urinary bladder, skin, and gastrointestinal tract. The mouse AQP3 gene was analyzed, and AQP3 null mice were generated by targeted gene disruption. The growth and phenotype of AQP3 null mice were grossly normal except for polyuria. AQP3 deletion had little effect on AQP1 or AQP4 protein expression but decreased AQP2 protein expression particularly in renal cortex. Fluid consumption in AQP3 null mice was more than 10-fold greater than that in wild-type litter mates, and urine osmolality (<275 milliosmol) was much lower than in wild-type mice (>1,200 milliosmol). After 1-desamino-8-d-arginine-vasopressin administration or water deprivation, the AQP3 null mice were able to concentrate their urine partially to approximately 30% of that in wild-type mice. Osmotic water permeability of cortical collecting-duct basolateral membrane, measured by a spatial filtering optics method, was >3-fold reduced by AQP3 deletion. To test the hypothesis that the residual concentrating ability of AQP3 null mice was due to the inner medullary collecting-duct water channel AQP4, AQP3/AQP4 double-knockout mice were generated. The double-knockout mice had greater impairment of urinary-concentrating ability than did the AQP3 single-knockout mice. Our findings establish a form of nephrogenic diabetes insipidus produced by impaired water permeability in collecting-duct basolateral membrane. Basolateral membrane aquaporins may thus provide blood-accessible targets for drug discovery of aquaretic inhibitors.
Figures
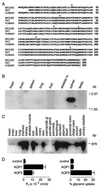
Figure 1
Analysis of mouse AQP3 tissue distribution and function. (A) Comparison of deduced amino acid sequences of mouse, rat, and human AQP3. Conserved residues are shown in bold. Arrows, intron positions; *, glycosylation site. (B) Multitissue Northern blot probed with full-length AQP3 coding sequence. (C) Multitissue PCR/Southern blot showing AQP3 transcript distribution. (D) Osmotic water and glycerol permeabilities of control (water-injected) and mouse AQP1- and AQP3-expressing Xenopus oocytes.
Figure 2
Generation of AQP3 null mice. (A Top) Organization and restriction map of the mouse AQP3 gene based on PCR analysis, restriction digestion, and DNA sequencing. Rectangles indicate exon segments that constitute coding (filled) and untranslated (open) sequences. (A Middle and Bottom) Targeting strategy for AQP3 gene deletion. Homologous recombination results in replacement of the indicated segments of the AQP3 gene by a 1.8-kb polII-neo selectable marker. The probe used for Southern blot analysis is indicated (labeled “probe”), and the 1.2-kb amplified region in PCR analysis is shown. (B) Southern blot of mouse liver genomic DNA digested with _Eco_RI and probed as indicated in A. (C) Northern blot of mouse kidney probed with the mouse AQP3 coding sequence. (D) Immunofluorescence localization of AQP3 protein in kidney cortex of wild-type (Upper) and AQP3 null (Lower) mouse.
Figure 3
Urinary-concentrating function in AQP3 single-knockout and AQP3/AQP4 double-knockout mice. (A) Fluid consumption (Upper) and urine output (Lower) over 24 h in mice of indicated genotype (SEM; 16 mice per genotype). (B) Urine osmolalities measured while mice were given free access to food and water before and after DDAVP administration and after a 36-h water deprivation (SEM; 12 mice per genotype). *, P < 0.005 compared with wild-type mouse.
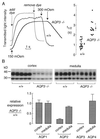
Figure 4
Effect of AQP3 deletion on collecting-duct water permeability and expression of renal aquaporins. (A) Osmotic water permeability in basolateral membrane of cortical collecting ducts. (A Left) Representative time courses of collecting-duct cell volume (genotype indicated). Perfusion with hypoosmolar solution (150 mosM) produced cell swelling and increased transmitted optical signal. Perfusion exchange time (labeled “add dye” and “remove dye”) was determined by absorbance of the dye Evans blue. (A Right) Averaged swelling rates for indicated mouse genotypes (SEM; *, P < 0.001). (B) Effect of AQP3 deletion on expression of renal aquaporin proteins. (B Upper) Immunoblot analysis of AQP2 in membranes from renal cortex and medulla from six wild-type and six AQP3-knockout mice. (B Lower) Quantitative densitometry of immunoblot data for indicated aquaporins.
Similar articles
- Erythrocyte water permeability and renal function in double knockout mice lacking aquaporin-1 and aquaporin-3.
Yang B, Ma T, Verkman AS. Yang B, et al. J Biol Chem. 2001 Jan 5;276(1):624-8. doi: 10.1074/jbc.M008664200. J Biol Chem. 2001. PMID: 11035042 - Aquaporin-4-Mediated Water Permeability Rescues Aquaporin-3 Deficiency Caused Nephrogenic Diabetes Insipidus.
Ying Y, Qiu Z, Liu J, Quan Y, An Y, Lu F, Wang K, Li M, Zhou H, Yang B. Ying Y, et al. Acta Physiol (Oxf). 2025 Jul;241(7):e70072. doi: 10.1111/apha.70072. Acta Physiol (Oxf). 2025. PMID: 40497427 - Fourfold reduction of water permeability in inner medullary collecting duct of aquaporin-4 knockout mice.
Chou CL, Ma T, Yang B, Knepper MA, Verkman AS. Chou CL, et al. Am J Physiol. 1998 Feb;274(2):C549-54. doi: 10.1152/ajpcell.1998.274.2.C549. Am J Physiol. 1998. PMID: 9486146 - Role of aquaporin water channels in kidney and lung.
Verkman AS. Verkman AS. Am J Med Sci. 1998 Nov;316(5):310-20. doi: 10.1097/00000441-199811000-00004. Am J Med Sci. 1998. PMID: 9822113 Review. - Renal concentrating and diluting function in deficiency of specific aquaporin genes.
Verkman AS. Verkman AS. Exp Nephrol. 2002;10(4):235-40. doi: 10.1159/000063697. Exp Nephrol. 2002. PMID: 12097826 Review.
Cited by
- Expression of aquaporin-3 in ipsilateral rat kidney with unilateral partial ureteral obstruction.
Lee JY, Shin JH, Song KH, Lim JS, Sul CK. Lee JY, et al. Korean J Urol. 2013 Apr;54(4):266-70. doi: 10.4111/kju.2013.54.4.266. Epub 2013 Apr 16. Korean J Urol. 2013. PMID: 23614066 Free PMC article. - Molecular physiology of urinary concentration defect in elderly population.
Kishore BK, Kran CM, Reif M, Menon AG. Kishore BK, et al. Int Urol Nephrol. 2001;33(2):235-48. doi: 10.1023/a:1015239915543. Int Urol Nephrol. 2001. PMID: 12092636 Review. - Aquaporin water channels in transepithelial fluid transport.
Tradtrantip L, Tajima M, Li L, Verkman AS. Tradtrantip L, et al. J Med Invest. 2009;56 Suppl(Suppl):179-84. doi: 10.2152/jmi.56.179. J Med Invest. 2009. PMID: 20224178 Free PMC article. - Renal water transport in health and disease.
Feraille E, Sassi A, Olivier V, Arnoux G, Martin PY. Feraille E, et al. Pflugers Arch. 2022 Aug;474(8):841-852. doi: 10.1007/s00424-022-02712-9. Epub 2022 Jun 9. Pflugers Arch. 2022. PMID: 35678906 Free PMC article. Review. - Role of Aquaporins during Teleost Gametogenesis and Early Embryogenesis.
Chauvigné F, Zapater C, Cerdà J. Chauvigné F, et al. Front Physiol. 2011 Sep 30;2:66. doi: 10.3389/fphys.2011.00066. eCollection 2011. Front Physiol. 2011. PMID: 21994496 Free PMC article.
References
- Ma T, Frigeri A, Hasegawa H, Verkman A S. J Biol Chem. 1994;269:21845–21849. - PubMed
- Yang B, Verkman A S. J Biol Chem. 1997;272:16140–16146. - PubMed
- Meinild A K, Klaerke D A, Zeuthen T. J Biol Chem. 1998;273:32446–32451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK035124/DK/NIDDK NIH HHS/United States
- R01 HL059198/HL/NHLBI NIH HHS/United States
- HL60288/HL/NHLBI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- HL59198/HL/NHLBI NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases