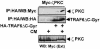The atypical PKC-interacting protein p62 channels NF-kappaB activation by the IL-1-TRAF6 pathway - PubMed (original) (raw)
The atypical PKC-interacting protein p62 channels NF-kappaB activation by the IL-1-TRAF6 pathway
L Sanz et al. EMBO J. 2000.
Abstract
The atypical protein kinase C (aPKC)-interacting protein, p62, has previously been shown to interact with RIP, linking these kinases to NF-kappaB activation by tumor necrosis factor alpha (TNFalpha). The aPKCs have been implicated in the activation of IKKbeta in TNFalpha-stimulated cells and have been shown to be activated in response to interleukin-1 (IL-1). Here we demonstrate that the inhibition of the aPKCs or the down-regulation of p62 severely abrogates NF-kappaB activation by IL-1 and TRAF6, suggesting that both proteins are critical intermediaries in this pathway. Consistent with this we show that p62 selectively interacts with the TRAF domain of TRAF6 but not that of TRAF5 or TRAF2 in co-transfection experiments. The binding of endogenous p62 to TRAF6 is stimulus dependent, reinforcing the notion that this is a physiologically relevant interaction. Furthermore, we demonstrate that the N-terminal domain of TRAF6, which is required for signaling, interacts with zetaPKC in a dimerization-dependent manner. Together, these results indicate that p62 is an important intermediary not only in TNFalpha but also in IL-1 signaling to NF-kappaB through the specific adapters RIP and TRAF6.
Figures
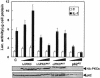
Fig. 1. Role of λ/ιPKC and p62 in NF–κB activation by IL–1. Subconfluent cultures of HepG2 cells were transfected with 1 ng of the κB-luciferase reporter gene plasmid along with 1 or 2 μg of HA–tagged versions of wild-type or a dominant-negative mutant of λ/ιPKC, an HA–tagged dominant-negative mutant of αPKC, or the antisense p62 plasmid (p62AS), and enough empty vector to give 2.5 μg of total DNA. After 24 h, cells were stimulated or not with IL–1β (1 ng/ml for 4 h) and extracts were prepared and the levels of luciferase activity were determined as described in Materials and methods. Results are the mean ± SD of three independent experiments with incubations in duplicate. The panels below show representative control blots of the expression of the HA–tagged constructs and p62 levels from one of the experiments.
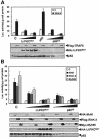
Fig. 2. Role of λ/ιPKC and p62 in NF–κB activation by TRAF6, IRAK and MyD88. Subconfluent cultures of 293 cells were transfected with 1 ng of the κB-luciferase reporter gene plasmid along with 0.1 μg of Flag-TRAF6 (A), HA–IRAK, Flag-IRAK2 or Myc-MyD88 (B) with or without 1 or 2 μg of HA–λ/ιPKC dominant-negative mutant (HA–λ/ιPKC_MUT_) (A and B), 2 μg of an expression vector for HA–p62 (A), or the antisense p62 plasmid (p62_AS_) (A and B), and enough empty vector to give 2.5 μg of total DNA. After 24 h, cell extracts were prepared and the levels of luciferase activity were determined. Results are the mean ± SD of three independent experiments with incubations in duplicate. The panels below show representative control blots of the expression of the differently tagged constructs and p62 levels from one of the experiments.
Fig. 3. Lack of a role for p62 and λ/ιPKC in TRAF6-induced JNK activation. Subconfluent cultures of 293 cells were transfected with 3 μg of Flag-TRAF6 with or without 6 or 10 μg of HA–λ/ιPKC dominant-negative mutant (HA–λ/ιPKC_MUT_) or the antisense p62 plasmid (p62_AS_), and enough empty vector to give 20 μg of total DNA. After 24 h, cell extracts were prepared and phospho-Jun was determined by immunoblot analysis with an anti-phospho-Jun antibody. Extracts were also immunoblotted with antibodies for the Flag and HA epitopes, as well as for p62. This is a representative experiment of another two with similar results.
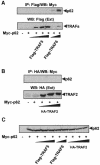
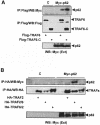
Fig. 4. Interaction in vivo of p62 with TRAF6 but not with TRAF2 or TRAF5. Subconfluent cultures of 293 cells in 100–mm-diameter plates were transfected with 4 μg of the Myc-p62 expression plasmid either alone or in combination with 15 or 20 μg of Flag-TRAF6, 2.5, 5 or 10 μg of Flag-TRAF5 (A) or 2, 4 or 6 μg of HA–TRAF2 (B) and enough pCDNA3 plasmid to give 25 μg of total DNA. After transfection (24 h), cell extracts were immunoprecipitated with anti–Flag (A) or anti-HA (B) antibodies, after which the immunoprecipitates were analyzed by immunoblotting with an anti-Myc antibody. An aliquot [one-tenth of the amount of extract (Ext) used for the immunoprecipitation] was loaded in the gels and analyzed by immunoblotting with the corresponding anti-tag antibodies. The gel shown in (C) demonstrates that the amount of Myc-p62 expressed was comparable in all the transfection points. Essentially identical results were obtained in another three independent experiments.
Fig. 5. ζPKC forms a ternary complex with p62 and TRAF6. Subconfluent cultures of 293 cells in 100–mm-diameter plates were transfected with 4 μg of Myc-p62 along with 5 μg of HA–ζPKC expression plasmid either alone or in combination with 20 μg of Flag-TRAF6 and enough pCDNA3 plasmid to give 30 μg of total DNA. After transfection (24 h), cell extracts were immunoprecipitated with anti-Myc or anti-Flag antibodies, after which the immunoprecipitates were analyzed by immunoblotting with anti-Flag, anti-Myc or anti-HA antibodies. An aliquot [one-tenth of the amount of extract (Ext) used for the immunoprecipitation] was loaded in the gels and analyzed by immunoblotting with the corresponding anti-tag antibodies. Essentially identical results were obtained in another two independent experiments.
Fig. 6. The interaction of endogenous p62 with TRAF6 is IL–1 dependent. (A) Subconfluent cultures of HepG2 cells in 100–mm-diameter plates were transfected with 25 μg of an expression vector for Flag-TRAF6. Twenty-four hours post-transfection, cells were either untreated or stimulated with IL–1 (1 ng/ml) for different times. Afterwards, cell extracts were immunoprecipitated with a pre-immune or an anti-p62 polyclonal antibody, and the immunoprecipitates were analyzed by immunoblotting with a monoclonal anti-Flag antibody. An aliquot [one-tenth of the amount of extract (Ext) used for the immunoprecipitation] was loaded in the gels and analyzed by immunoblotting with the anti-Flag antibody. Essentially identical results were obtained in another three independent experiments. (B) Subconfluent cultures of HepG2 cells in 100–mm-diameter plates stimulated or not with IL–1 (1 ng/ml) for different times. Afterwards, cell extracts were immunoprecipitated with the polyclonal anti-p62 antibody, and the immunoprecipitates were analyzed by immuno- blotting with the monoclonal anti-TRAF6 and anti-p62 antibodies. Essentially identical results were obtained in another two independent experiments.
Fig. 7. TRAF6 links p62 to IRAK. Subconfluent cultures of HepG2 cells in 100–mm-diameter plates were transfected with 4 μg of the Myc-p62 expression plasmid either alone or in combination with 4 μg of HA–IRAK with or without 15 μg of Flag-TRAF6 and enough pCDNA3 plasmid to give 25 μg of total DNA. After transfection (24 h), cells were either untreated or stimulated with IL–1 for 10 min. Cell extracts were immunoprecipitated with an anti-Myc antibody, after which the immunoprecipitates were analyzed by immunoblotting with an anti-HA antibody. An aliquot [one-tenth of the amount of extract (Ext) used for the immunoprecipitation] was loaded in the gels and analyzed by immunoblotting with the corresponding anti-tag antibodies. Essentially identical results were obtained in another two independent experiments.
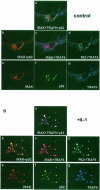
Fig. 8. Colocalization of p62 with TRAF6 and IRAK in IL–1-stimulated cells. HepG2 cells were transfected with Flag-TRAF6 and HA–IRAK along with a GFP–p62 construct, after which cells were either untreated (A) or stimulated with IL–1 for 10 min (B). Cells were then analyzed by triple confocal laser scanning microscopy with a rabbit polyclonal anti-HA antibody and an anti-rabbit Alexa 594 antibody (red fluorescence) to detect IRAK, and a monoclonal anti-Flag antibody and an anti-mouse Cy5 antibody (blue fluorescence) to detect TRAF6. Green fluorescence indicates the expression of GFP–p62. Essentially identical results were obtained in another two independent experiments.
Fig. 9. Schematic representation of the various constructs used for the mapping of the p62–TRAF6 and p62–RIP interaction domains. Constructs were prepared as described in Materials and methods. AS, acidic sequence and ζPKC-interacting region; ZZ, zinc-finger domain.
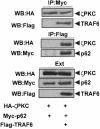
Fig. 10. Simultaneous interaction of p62 with TRAF6 and RIP. Subconfluent cultures of 293 cells in 100–mm-diameter plates were transfected with 4 μg of HA–p62 expression plasmid along with 5 μg of Flag-RIP either alone or in combination with 5, 10 or 15 μg of Flag-TRAF6 and enough pCDNA3 plasmid to give 25 μg of total DNA. After transfection (24 h), cell extracts were immunoprecipitated with anti-HA antibody, after which the immunoprecipitates were analyzed by immunoblotting with anti-Flag antibody. An aliquot [one-tenth of the amount of extract (Ext) used for the immunoprecipitation] was loaded in the gels and analyzed by immunoblotting with the corresponding anti-tag antibodies. Essentially identical results were obtained in another three independent experiments.
Fig. 11. Interaction of p62 with the TRAF domain of TRAF6. Subconfluent cultures of 293 cells were transfected with expression plasmids for Flag-tagged wild-type TRAF6 (15 μg) or a mutant in which the N–terminal domain of TRAF6 was deleted (20 μg; TRAF6–C) (A), or with HA–TRAF2 (7.5 μg) or 25 μg of the HA–tagged chimeric constructs TRAF2/6 or TRAF6/2 (B) along with 4 μg of a control vector or a Myc-p62 expression plasmid. Cell extracts were immunoprecipitated with anti-Flag (A) or anti-HA (B) antibodies and the immunoprecipitates were analyzed by immunoblotting with anti-Myc (A and B), anti-Flag (A) or anti-HA (B) antibodies. An aliquot [one-tenth of the amount of extract (Ext) used for the immunoprecipitation] was loaded in the gels and analyzed by immunoblotting with the anti-Myc antibody. Essentially identical results were obtained in another three independent experiments.
Fig. 12. The N–terminal domain of TRAF6 interacts with ζPKC in a dimerization-dependent manner. Subconfluent cultures of 293 cells in 100–mm-diameter plates were transfected with 10 μg of either pCDNA3 or an expression plasmid for the HA–TRAF6ΔC-Gyr construct along with 7.5 μg of a Myc-ζPKC expression vector. After transfection (24 h), cells were stimulated or not with CM for 15 min, after which cell extracts were immunoprecipitated with an anti-HA antibody and the immunoprecipitates were analyzed with anti-Myc and anti-HA antibodies. An aliquot [one-tenth of the amount of extract (Ext) used for the immunoprecipitation] was loaded in the gels and analyzed by immunoblotting with the anti-Myc antibody. Essentially identical results were obtained in another three independent experiments.
Similar articles
- The interaction of p62 with RIP links the atypical PKCs to NF-kappaB activation.
Sanz L, Sanchez P, Lallena MJ, Diaz-Meco MT, Moscat J. Sanz L, et al. EMBO J. 1999 Jun 1;18(11):3044-53. doi: 10.1093/emboj/18.11.3044. EMBO J. 1999. PMID: 10356400 Free PMC article. - TRAF6 is a signal transducer for interleukin-1.
Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. Cao Z, et al. Nature. 1996 Oct 3;383(6599):443-6. doi: 10.1038/383443a0. Nature. 1996. PMID: 8837778 - NF-kappa B activation by tumor necrosis factor and interleukin-1.
Cao Z, Tanaka M, Regnier C, Rothe M, Yamit-hezi A, Woronicz JD, Fuentes ME, Durnin MH, Dalrymple SA, Goeddel DV. Cao Z, et al. Cold Spring Harb Symp Quant Biol. 1999;64:473-83. doi: 10.1101/sqb.1999.64.473. Cold Spring Harb Symp Quant Biol. 1999. PMID: 11232324 Review. No abstract available. - [Interleukin 1 signal transduction].
Yoshida Y, Yamashita U. Yoshida Y, et al. J UOEH. 2003 Jun 1;25(2):237-48. doi: 10.7888/juoeh.25.237. J UOEH. 2003. PMID: 12813866 Review. Japanese.
Cited by
- p62/SQSTM1 by Binding to Vitamin D Receptor Inhibits Hepatic Stellate Cell Activity, Fibrosis, and Liver Cancer.
Duran A, Hernandez ED, Reina-Campos M, Castilla EA, Subramaniam S, Raghunandan S, Roberts LR, Kisseleva T, Karin M, Diaz-Meco MT, Moscat J. Duran A, et al. Cancer Cell. 2016 Oct 10;30(4):595-609. doi: 10.1016/j.ccell.2016.09.004. Cancer Cell. 2016. PMID: 27728806 Free PMC article. - Interferon regulatory factor 7 is activated by a viral oncoprotein through RIP-dependent ubiquitination.
Huye LE, Ning S, Kelliher M, Pagano JS. Huye LE, et al. Mol Cell Biol. 2007 Apr;27(8):2910-8. doi: 10.1128/MCB.02256-06. Epub 2007 Feb 12. Mol Cell Biol. 2007. PMID: 17296724 Free PMC article. - PB1 domain interaction of p62/sequestosome 1 and MEKK3 regulates NF-kappaB activation.
Nakamura K, Kimple AJ, Siderovski DP, Johnson GL. Nakamura K, et al. J Biol Chem. 2010 Jan 15;285(3):2077-89. doi: 10.1074/jbc.M109.065102. Epub 2009 Nov 10. J Biol Chem. 2010. PMID: 19903815 Free PMC article. - Autophagy Mediates Astrogenesis in Adult Hippocampal Neural Stem Cells.
Ha S, Jeong SH, Yi K, Chu JJ, Kim S, Kim EK, Yu SW. Ha S, et al. Exp Neurobiol. 2019 Apr;28(2):229-246. doi: 10.5607/en.2019.28.2.229. Epub 2019 Apr 30. Exp Neurobiol. 2019. PMID: 31138991 Free PMC article. - The Deubiquitinating Enzyme USP20 Regulates the TNFα-Induced NF-κB Signaling Pathway through Stabilization of p62.
Ha J, Kim M, Seo D, Park JS, Lee J, Lee J, Park SH. Ha J, et al. Int J Mol Sci. 2020 Apr 28;21(9):3116. doi: 10.3390/ijms21093116. Int J Mol Sci. 2020. PMID: 32354117 Free PMC article.
References
- Akiba H. et al. (1998)CD27, a member of the tumor necrosis factor receptor superfamily, activates NF–κB and stress-activated protein kinase/c-Jun N–terminal kinase via TRAF2, TRAF5 and NF–κB-inducing kinase. J. Biol. Chem., 273, 13353–13358. - PubMed
- Arch R.H., Gedrich, R.W. and Thompson, C.B. (1998) Tumor necrosis factor receptor-associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev., 12, 2821–2830. - PubMed
- Berra E., Diaz–Meco, M.T., Dominguez, I., Municio, M.M., Sanz, L., Lozano, J., Chapkin, R.S. and Moscat, J. (1993) Protein kinase C ζ isoform is critical for mitogenic signal transduction. Cell, 74, 555–563. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous