Imaging constitutive exocytosis with total internal reflection fluorescence microscopy - PubMed (original) (raw)
Imaging constitutive exocytosis with total internal reflection fluorescence microscopy
J Schmoranzer et al. J Cell Biol. 2000.
Abstract
Total internal reflection fluorescence microscopy has been applied to image the final stage of constitutive exocytosis, which is the fusion of single post-Golgi carriers with the plasma membrane. The use of a membrane protein tagged with green fluorescent protein allowed the kinetics of fusion to be followed with a time resolution of 30 frames/s. Quantitative analysis allowed carriers undergoing fusion to be easily distinguished from carriers moving perpendicularly to the plasma membrane. The flattening of the carriers into the plasma membrane is seen as a simultaneous rise in the total, peak, and width of the fluorescence intensity. The duration of this flattening process depends on the size of the carriers, distinguishing small spherical from large tubular carriers. The spread of the membrane protein into the plasma membrane upon fusion is diffusive. Mapping many fusion sites of a single cell reveals that there are no preferred sites for constitutive exocytosis in this system.
Figures
Figure 1
Comparison of epi- and TIR illumination. A COS cell transfected with VSVG-GFP was imaged using (A) epi- and (B) TIR illumination.
Figure 5
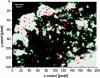
Map of fusion sites of a single cell. The exocytotic events (n = 147) from a single cell were superimposed as red dots onto a thresholded gray scale image of VSVG-GFP in the plasma membrane (see Materials and Methods). One edge of the cell is in the upper left. Under epi-illumination, the Golgi complex appeared in the lower right corner. Pixel size, 268 nm.
Figure 2
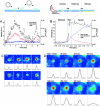
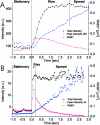
Analysis of carriers. The VSVG-GFP fluorescence was imaged for carriers close to the plasma membrane. Selected frames are shown from a sequence in (C) for a carrier which moved perpendicular to the coverslip, without fusing to the plasma membrane and in (D) for a carrier which fused to the plasma membrane. The intensity of the VSVG-GFP in frames C and D is shown in pseudocolor. Each sequence was processed with a running average in time of (±1 frame) and thresholded separately to aid visualization. The radially symmetric Gauss fit of the carrier fluorescence is shown below each frame. (A and B) The total intensity, peak intensity and the square of the Gaussian width for the carriers shown in C and D were plotted over time in A and B, respectively. The numbered arrows refer to the frames from sequences C and D. In B, the three phases, stationary, rise and spread, are separated by dotted lines. Times are marked relative to the start of the rise phase.
Figure 3
Selected frames from a sequence showing the transport, docking, and fusion of a tubular carrier. Times are marked relative to the start of the rise phase.
Figure 4
Carrier fusion events with different rise times. Total intensity, peak intensity, and square of the Gaussian width were plotted on the same time axis for carriers with long and short rise times (time is marked relative to the start of the rise phase). (A) A carrier with a long rise time (∼1.9 s) and tubular morphology (taken from the sequence shown in Fig. 3). (B) A carrier with short rise time (∼160 ms) and spotlike morphology. A similar example is shown in Fig. 2 D. The three phases, stationary, rise and spread, are separated by dotted lines.
Similar articles
- Fusion of constitutive membrane traffic with the cell surface observed by evanescent wave microscopy.
Toomre D, Steyer JA, Keller P, Almers W, Simons K. Toomre D, et al. J Cell Biol. 2000 Apr 3;149(1):33-40. doi: 10.1083/jcb.149.1.33. J Cell Biol. 2000. PMID: 10747085 Free PMC article. - Visualization of regulated exocytosis with a granule-membrane probe using total internal reflection microscopy.
Allersma MW, Wang L, Axelrod D, Holz RW. Allersma MW, et al. Mol Biol Cell. 2004 Oct;15(10):4658-68. doi: 10.1091/mbc.e04-02-0149. Epub 2004 Jul 28. Mol Biol Cell. 2004. PMID: 15282339 Free PMC article. - Golgi network targeting and plasma membrane internalization signals in vaccinia virus B5R envelope protein.
Ward BM, Moss B. Ward BM, et al. J Virol. 2000 Apr;74(8):3771-80. doi: 10.1128/jvi.74.8.3771-3780.2000. J Virol. 2000. PMID: 10729152 Free PMC article. - Exocytotic vesicle behaviour assessed by total internal reflection fluorescence microscopy.
Burchfield JG, Lopez JA, Mele K, Vallotton P, Hughes WE. Burchfield JG, et al. Traffic. 2010 Apr;11(4):429-39. doi: 10.1111/j.1600-0854.2010.01039.x. Epub 2010 Jan 12. Traffic. 2010. PMID: 20070611 Review. - Genesis of polarity in renal tubular cells.
Rodriguez-Boulan E. Rodriguez-Boulan E. Miner Electrolyte Metab. 1986;12(1):20-4. Miner Electrolyte Metab. 1986. PMID: 3007959 Review.
Cited by
- CRAC channels regulate astrocyte Ca2+ signaling and gliotransmitter release to modulate hippocampal GABAergic transmission.
Toth AB, Hori K, Novakovic MM, Bernstein NG, Lambot L, Prakriya M. Toth AB, et al. Sci Signal. 2019 May 21;12(582):eaaw5450. doi: 10.1126/scisignal.aaw5450. Sci Signal. 2019. PMID: 31113852 Free PMC article. - Vesicular transport of progeny parvovirus particles through ER and Golgi regulates maturation and cytolysis.
Bär S, Rommelaere J, Nüesch JP. Bär S, et al. PLoS Pathog. 2013 Sep;9(9):e1003605. doi: 10.1371/journal.ppat.1003605. Epub 2013 Sep 19. PLoS Pathog. 2013. PMID: 24068925 Free PMC article. - Polarization-controlled TIRFM with focal drift and spatial field intensity correction.
Johnson DS, Toledo-Crow R, Mattheyses AL, Simon SM. Johnson DS, et al. Biophys J. 2014 Mar 4;106(5):1008-19. doi: 10.1016/j.bpj.2013.12.043. Biophys J. 2014. PMID: 24606926 Free PMC article. - Partial internal reflections on total internal reflection fluorescent microscopy.
Simon SM. Simon SM. Trends Cell Biol. 2009 Nov;19(11):661-8. doi: 10.1016/j.tcb.2009.08.003. Epub 2009 Oct 7. Trends Cell Biol. 2009. PMID: 19818624 Free PMC article. - Plasma membrane is the site of productive HIV-1 particle assembly.
Jouvenet N, Neil SJ, Bess C, Johnson MC, Virgen CA, Simon SM, Bieniasz PD. Jouvenet N, et al. PLoS Biol. 2006 Dec;4(12):e435. doi: 10.1371/journal.pbio.0040435. PLoS Biol. 2006. PMID: 17147474 Free PMC article.
References
- Albillos A., Dernick G., Horstmann H., Almers W., De Toledo G.A., Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. - PubMed
- Ales E., Tabares L., Poyato J.M., Valero V., Lindau M., Alvarez D.T. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat. Cell Biol. 1999;1:40–44. - PubMed
- Artalejo C.R., Elhamdani A., Palfrey H.C. Secretiondense-core vesicles can kiss-and-run too. Curr. Biol. 1998;8:R62–R65. - PubMed
- Axelrod D. Total internal reflection fluorescence microscopy. Methods Cell Biol. 1989;30:245–270. - PubMed
- Axelrod D., Hellen E.H., Fulbright R.M. Total internal reflection fluorescence. In: Lakowicz J.R., editor. Topics in Fluorescence Spectroscopy, Volume 3Biochemical Applications. Plenum Press; New York: 1992. pp. 289–343.