GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex - PubMed (original) (raw)
GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex
E C Dell'Angelica et al. J Cell Biol. 2000.
Abstract
Formation of intracellular transport intermediates and selection of cargo molecules are mediated by protein coats associated with the cytosolic face of membranes. Here, we describe a novel family of ubiquitous coat proteins termed GGAs, which includes three members in humans and two in yeast. GGAs have a modular structure consisting of a VHS domain, a region of homology termed GAT, a linker segment, and a region with homology to the ear domain of gamma-adaptins. Immunofluorescence microscopy showed colocalization of GGAs with Golgi markers, whereas immunoelectron microscopy of GGA3 revealed its presence on coated vesicles and buds in the area of the TGN. Treatment with brefeldin A or overexpression of dominant-negative ADP ribosylation factor 1 (ARF1) caused dissociation of GGAs from membranes. The GAT region of GGA3 was found to: target a reporter protein to the Golgi complex; induce dissociation from membranes of ARF-regulated coats such as AP-1, AP-3, AP-4, and COPI upon overexpression; and interact with activated ARF1. Disruption of both GGA genes in yeast resulted in impaired trafficking of carboxypeptidase Y to the vacuole. These observations suggest that GGAs are components of ARF-regulated coats that mediate protein trafficking at the TGN.
Figures
Figure 1
Schematic representation of GGAs and related proteins. Total numbers of amino acid residues of each protein are noted on the right. Specific domains or regions of homology are color coded. Black lines denote variable regions with no significant homology. Accession numbers are as follows: human GGA1, AF218584; human GGA2 (A-735G6.4), AC002400; human GGA3 (long isoform), AF219138; yeast Gga1p (YDR358w), NC_001136; yeast Gga2p (YHR108w), NC_001140; human TOM1, AJ006973; human TOM1-L1, AJ010071; human Hrs, D84064; yeast Vps27p, U24218; human STAM1, U43899; human γ1-adaptin, AB015317; human γ2-adaptin, AB015318.
Figure 2
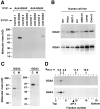
Biochemical characterization of endogenous GGA2 and GGA3. A, HeLa cells were metabolically labeled with [35S]methionine for ∼22 h and lysed in the presence of Triton X-100 (Dell'Angelica et al. 1997). The cleared extract was immunoprecipitated using antibodies to GGA2 or GGA3, and the resulting precipitates were subjected to a second immunoprecipitation step using antibodies to GGA2, GGA3, or two irrelevant proteins. The final immunoprecipitates were analyzed by 4–20% SDS-PAGE and fluorography. B, The indicated human cell lines were labeled with [35S]methionine and subjected to immunoprecipitation–recapture, using antibodies to GGA2 (top) or GGA3 (bottom). C, A detergent-free extract (Dell'Angelica et al. 1997) of metabolically labeled HeLa cells was centrifuged first at 600 g for 5 min to remove nuclei and then at 120,000 g for 90 min to obtain cytosolic (C) and post-nuclear membrane (M) fractions. Both fractions were subjected to immunoprecipitation–recapture with antibodies to GGA2 or GGA3. D, A cytosolic fraction obtained as in C was loaded onto a 4–20% sucrose gradient and centrifuged in an SW-41 Beckman rotor at 39,000 rpm for 17 h at 4°C. Collected fractions were analyzed for GGA2 and GGA3 by immunoprecipitation–recapture. The positions of protein standards and the AP-4 complex in the gradient are indicated by arrows and an arrowhead, respectively.
Figure 3
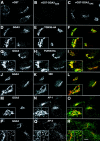
Intracellular localization of endogenous GGA3 analyzed by indirect immunofluorescence microscopy. A–C, HeLa cells were fixed, permeabilized, and incubated with rabbit antibody to the GAE domain of GGA3 in the presence of excess GST (A), GST-GGA3GAE (B), or GST-GGA2GAE (C) proteins. The bound antibody was revealed by staining with Cy3-conjugated donkey anti–rabbit IgG. D–R, HeLa cells transfected with HA-tagged TGN38 (D–F) or HA-tagged furin constructs (G–I), or untransfected HeLa cells (J–R) were fixed, permeabilized, and double-labeled with rabbit antibody to GGA3 followed by Cy3-conjugated donkey anti-rabbit IgG (D, G, J, M, and P; red channel), and mouse antibodies to the HA epitope (E and H), the 58K-Golgi protein (K), the γ1-adaptin subunit of AP-1 (N,100/3), or the AP-3 complex (Q, mouse polyclonal antibody to AP-3), followed by Alexa-488–conjugated donkey anti–mouse IgG (green channel). Stained cells were examined by confocal fluorescence microscopy. The third picture on each row in D–R was generated by merging of the images in the red and green channels; yellow indicates overlapping localization. Insets in D–O and the left inset in P–R show twofold magnified views of the Golgi region of the cells. The right inset in P–R shows a twofold magnified view of the peripheral cytoplasm. Bar, 10 μm.
Figure 4
Ultrastructural analysis of GGA3 localization by immunoelectron microscopy. Ultrathin frozen sections of HeLa cells transfected with a GGA3 construct were immunolabeled for GGA3 (10-nm gold; A and B) or for GGA3 (5-nm gold) and TGN46 (10-nm gold; C), as described in Materials and Methods. N, nucleus; G, Golgi stack; ER, endoplasmic reticulum; PM, plasma membrane. Bars: (A) 0.2 μm; (B and C) 0.1 μm.
Figure 5
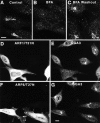
Effects of BFA and dominant-negative ARF mutants on the localization of endogenous GGA3 analyzed by immunofluorescence microscopy. A–C, Untreated HeLa cells (A), HeLa cells treated for 5 min at 37°C with 2 μg/ml BFA (B), or HeLa cells treated for 5 min with 2 μg/ml BFA followed by removal of the drug for 1 h at 37°C (C) were fixed, permeabilized, and incubated with rabbit antibody to GGA3, followed by Cy3-conjugated donkey anti–rabbit IgG. D–G, HeLa cells were transfected with HA-tagged ARF1/T31N (D and E) or HA-tagged ARF6/T27N (F and G). Cells were fixed, permeabilized, and double-labeled with rabbit antibody to GGA3 followed by Cy3-conjugated donkey anti–rabbit IgG (E and G), and mouse antibody to the HA epitope followed by Alexa-488–conjugated donkey anti–mouse IgG (D and F). Stained cells were examined by confocal fluorescence microscopy. Bars, 10 μm.
Figure 6
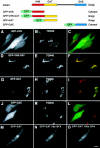
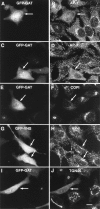
Intracellular localization of GFP fusion proteins containing different regions of GGA3. Top, Schematic representation of the GFP fusion proteins used in these experiments, including a summary of their localizations. A–L, Fluorescence microscopy of cells expressing GFP fusion proteins and immunostained for TGN46. HeLa cells were transfected with plasmids encoding GFP-VHS (A–C), GFP-VHS-GAT (D–F), GFP-GAT (G–I), or GFP-GAE (J–L). Transfected cells were fixed, permeabilized, and immunostained with a rabbit antibody to TGN46 followed by Cy3-conjugated donkey anti–rabbit IgG. Cells were examined by confocal fluorescence microscopy. A, D, G, and J, GFP fluorescence (green); B, E, H, and K, TGN46 (red). M–O, Live cells expressing GFP-GAT were imaged at 37°C by confocal fluorescence microscopy immediately before (M), and 56 s (N) and 100 s (O) after addition of 10 μg/ml BFA. Bar, 10 μm.
Figure 7
Effects of overexpression of GFP-GAT or GFP-VHS on protein coats and TGN46 analyzed by fluorescence microscopy. HeLa cells were transfected with plasmids encoding GFP fused to either the GAT (A–F, I and J) or VHS (G and H) regions of GGA3. Cells were fixed, permeabilized, and incubated with the 100/3 antibody to the γ1-adaptin subunit of AP-1 (B and H), the β3C1 antibody to the β3A subunit of AP-3 (D), or rabbit antibodies to the β-COP subunit of COPI (F) or TGN46 (J). Bound antibodies were revealed by staining with either Cy3-conjugated donkey anti–mouse IgG (B and H) or Cy3-conjugated donkey anti–rabbit IgG (D, F, and J). A, C, E, and I, GFP-GAT; G, GFP-VHS. Arrows point to cells that overexpress the GFP constructs. Bar, 10 μm.
Figure 8
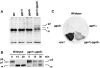
Binding of activated ARF1 to the GAT domain of GGA3. A, Two-hybrid assays. Yeast transformants expressing the combinations of constructs indicated in the figure were spotted onto plates lacking leucine and tryptophan, with or without histidine (+His and −His, respectively), and in the absence or presence of 10 mM 3AT. Filter β-galactosidase (β-gal) assays were performed on cells grown in the presence of histidine. Notice the growth in the absence of histidine and the positive β-galactosidase activity of transformants expressing the activated ARF1 mutant (Q71L) and the GGA3 constructs that include the GAT domain. B, In vitro binding assays. Bovine brain cytosol and recombinant ARF1/Q71L were either treated with 0.1 mM GTPγS (+GTPγS) or mock-treated (–GTPγS), and subsequently incubated with glutathione-Sepharose beads containing GST or GST-fusion proteins comprising the VHS, VHS-GAT, or GAE domains of human GGA3. Bound proteins were analyzed by immunoblotting, using the 1D9 antibody to ARF or antibodies to the Rab4 and dynamin I GTP-binding proteins. Notice the GTPγS-dependent binding of bovine brain ARF, and of recombinant ARF1/Q71L, to the GST-fusion protein bearing the VHS-GAT segment, but not the VHS or GAE regions, of GGA3.
Figure 9
Effects of disruption of yeast GGA genes on CPY processing and secretion. A, Immunoblot analysis of CPY in whole-cell lysates of wild-type, _gga1_Δ, _gga2_Δ, and _gga1_Δ/_gga2_Δ strains. The positions of the p2 and m forms of CPY are indicated. The asterisk indicates an unidentified intermediate species. B, Wild-type and _gga1_Δ/_gga2_Δ strains were pulse-labeled with [35S]methionine for 10 min at 30°C and then chased for 0, 15, or 30 min at 30°C. CPY was isolated by immunoprecipitation and analyzed by SDS-PAGE and fluorography. The positions of the p1, p2, and m forms of CPY are indicated. C, Colony blotting analysis of CPY secretion. Nitrocellulose replicas of streaks of the strains mentioned in A and of a vps1 mutant strain (positive control) were probed with anti-CPY antiserum to reveal secreted CPY.
Similar articles
- A family of proteins with gamma-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome.
Hirst J, Lui WW, Bright NA, Totty N, Seaman MN, Robinson MS. Hirst J, et al. J Cell Biol. 2000 Apr 3;149(1):67-80. doi: 10.1083/jcb.149.1.67. J Cell Biol. 2000. PMID: 10747088 Free PMC article. - GGAs: roles of the different domains and comparison with AP-1 and clathrin.
Hirst J, Lindsay MR, Robinson MS. Hirst J, et al. Mol Biol Cell. 2001 Nov;12(11):3573-88. doi: 10.1091/mbc.12.11.3573. Mol Biol Cell. 2001. PMID: 11694590 Free PMC article. - ADP-ribosylation factor (ARF) interaction is not sufficient for yeast GGA protein function or localization.
Boman AL, Salo PD, Hauglund MJ, Strand NL, Rensink SJ, Zhdankina O. Boman AL, et al. Mol Biol Cell. 2002 Sep;13(9):3078-95. doi: 10.1091/mbc.e02-02-0078. Mol Biol Cell. 2002. PMID: 12221117 Free PMC article. - The structure and function of GGAs, the traffic controllers at the TGN sorting crossroads.
Nakayama K, Wakatsuki S. Nakayama K, et al. Cell Struct Funct. 2003 Oct;28(5):431-42. doi: 10.1247/csf.28.431. Cell Struct Funct. 2003. PMID: 14745135 Review. - The GGA proteins: key players in protein sorting at the trans-Golgi network.
Ghosh P, Kornfeld S. Ghosh P, et al. Eur J Cell Biol. 2004 Jul;83(6):257-62. doi: 10.1078/0171-9335-00374. Eur J Cell Biol. 2004. PMID: 15511083 Review.
Cited by
- GGA1 interacts with the endosomal Na+/H+ exchanger NHE6 governing localization to the endosome compartment.
Ma L, Kasula RK, Ouyang Q, Schmidt M, Morrow EM. Ma L, et al. J Biol Chem. 2024 Aug;300(8):107552. doi: 10.1016/j.jbc.2024.107552. Epub 2024 Jul 11. J Biol Chem. 2024. PMID: 39002678 Free PMC article. - A family of proteins with gamma-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome.
Hirst J, Lui WW, Bright NA, Totty N, Seaman MN, Robinson MS. Hirst J, et al. J Cell Biol. 2000 Apr 3;149(1):67-80. doi: 10.1083/jcb.149.1.67. J Cell Biol. 2000. PMID: 10747088 Free PMC article. - Laa1p, a conserved AP-1 accessory protein important for AP-1 localization in yeast.
Fernández GE, Payne GS. Fernández GE, et al. Mol Biol Cell. 2006 Jul;17(7):3304-17. doi: 10.1091/mbc.e06-02-0096. Epub 2006 May 10. Mol Biol Cell. 2006. PMID: 16687571 Free PMC article. - Membrane-trafficking sorting hubs: cooperation between PI4P and small GTPases at the trans-Golgi network.
Santiago-Tirado FH, Bretscher A. Santiago-Tirado FH, et al. Trends Cell Biol. 2011 Sep;21(9):515-25. doi: 10.1016/j.tcb.2011.05.005. Epub 2011 Jul 19. Trends Cell Biol. 2011. PMID: 21764313 Free PMC article. Review. - The yeast endocytic early/sorting compartment exists as an independent sub-compartment within the _trans_-Golgi network.
Toshima JY, Tsukahara A, Nagano M, Tojima T, Siekhaus DE, Nakano A, Toshima J. Toshima JY, et al. Elife. 2023 Jul 21;12:e84850. doi: 10.7554/eLife.84850. Elife. 2023. PMID: 37477116 Free PMC article.
References
- Asao H., Sasaki Y., Arita T., Tanaka N., Endo K., Kasai H., Takeshita T., Endo Y., Fujita T., Sugamura K. Hrs is associated with STAM, a signal-transducing adaptor molecule. Its suppressive effect on cytokine-induced cell growth. J. Biol. Chem. 1997;272:32785–32791. - PubMed
- Barr F.A. A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins. Curr. Biol. 1999;9:381–384. - PubMed
- Berben G., Dumont J., Gilliquet V., Bolle P.A., Hilger F. The YDp plasmidsa uniform set of vectors bearing versatile gene disruption cassettes for Saccharomyces cerevisiae . Yeast. 1991;7:475–477. - PubMed
- Boman A.L., Kahn R.A. Arf proteinsthe membrane traffic police? Trends Biochem. Sci. 1995;20:147–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous