Disruption of T cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor beta II receptor - PubMed (original) (raw)
Disruption of T cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor beta II receptor
P J Lucas et al. J Exp Med. 2000.
Abstract
The immune system, despite its complexity, is maintained at a relative steady state. Mechanisms involved in maintaining lymphocyte homeostasis are poorly understood; however, recent availability of transgenic (Tg) and knockout mouse models with altered balance of lymphocyte cell populations suggest that cytokines play a major role in maintaining lymphocyte homeostasis. We show here that transforming growth factor (TGF)-beta plays a critical role in maintaining CD8(+) T cell homeostasis in a Tg mouse model that specifically overexpresses a dominant negative TGF-beta II receptor (DNRII) on T cells. DNRII T cell Tg mice develop a CD8(+) T cell lymphoproliferative disorder resulting in the massive expansion of the lymphoid organs. These CD8(+) T cells are phenotypically "naive" except for the upregulation of the cell surface molecule CD44, a molecule usually associated with memory T cells. Despite their dominance in the peripheral lymphoid organs, CD8(+) T cells appear to develop normally in the thymus, suggesting that TGF-beta exerts its homeostatic control in the peripheral immune system.
Figures
Figure 1
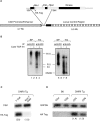
Construction and expression of the DNRII transgene. (A) Schematic representation of the expression vector with transgene. (B) Association of TGF-β1 with DNRII. Either splenocytes (SP) from C57BL/6 mice or TG1124 cells (TG) were affinity labeled with 125I–TGF-β1 in the absence (−) or presence (+) of cold TGF-β1. Cell lysates were subjected to immunoprecipitation by either anti–TGF-β II receptor, specific for cytoplasmic tail of endogenous TGF-β II, or anti-HA tag, specific for DNRII. Immunoprecipitation of receptors was analyzed by SDS-PAGE and autoradiography. (C) Semiquantitative RT-PCR to establish tissue-specific expression of DNRII transgene. CSK, a ubiquitously expressed kinase, was used to establish relative expression of the transgene in T cell and non-T cell populations (number in parentheses). (D) Semiquantitative RT-PCR to establish expression within T cell subsets. Expression of G3PDH was used to calculate relative expression between CD4+ and CD8+ T cell subsets (number in parentheses).
Figure 2
Small lymphocyte infiltration in DNRII Tg founder T12H1. (A) Extensive perivascular infiltration of small lymphocytes into the lung with little inflammation. (B) Higher magnification showing lymphoid cells in alveolar walls. (C) Spleen containing large white pulp areas with disruption of normal architecture. (D) Higher magnification showing a uniform population of lymphoid cells. (E) Liver with extensive perivascular and periportal lymphoid infiltration, with many lymphoid cells present in the blood but little inflammation. (F) Higher magnification showing infiltration around the blood vessel and in the sinusoids. Magnifications: A, 75×; C and E, 50×; B, D, and F, 150×.
Figure 3
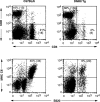
Flow cytometric analysis of B and T cells in DNRII Tg mice. Splenocytes from 12-wk-old founder T12H1 (right panels) or an aged-matched C57BL/6 mouse (left panels) were analyzed for expression of T cell markers, CD4 and CD8 (top panels), and B cell markers, B220 and MHC class II (CII) molecules (bottom panels). Absolute cell numbers ×10−6 shown in parentheses. At least 10,000 events were collected for each group.
Figure 4
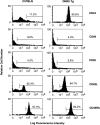
T cell activation marker expression on surfaces of DNRII Tg CD8+ T cells. LN T cells from 27-wk-old non-Tg littermate mouse (left histograms) or DNRII Tg mouse I2A1 (right histograms) were stained with Abs specific for a panel of T cell activation/memory markers. At least 10,000 events were collected for each group.
Figure 5
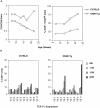
Time course analysis of CD8+ peripheral T cells in DNRII Tg mice. (A) PBLs from founder line E1A3 (□) or non-Tg littermate mice (○) were analyzed by flow cytometry for T cell markers CD4 and CD8 (left; expressed as CD4/CD8 ratio) and CD8+CD44hi surface marker (right) over time. (B) TCR V-β repertoire was measured by a panel of TCR V-β–specific mAbs. Percentage of CD8+ PBLs expressing a specific TCR V-β receptor was plotted over time. At least 10,000 events were collected per time point.
Figure 6
Thymic development and cell cycle analysis of DNRII Tg T cells. (A) LN cells (top panels) or thymocytes (bottom panels) from 27-wk-old DNRII Tg line I2A1 (right panels) or littermate control mice (left panels) were stained with anti-CD4 and -CD8 mAbs and were detected by flow cytometric analysis. (B) CD8+ LN T cells (four leftmost panels) or thymocytes (right panels) from 19-wk-old DNRII Tg line I2C2 (center panels) or littermate control mice (left and right panels) were stained with propidium iodide (PI). Percentage of cells in cycle (S/M/G2) is indicated as percentage of incorporation of PI as detected by flow cytometric analysis. Thymocytes (right panels) were used as a positive control for the detection of cycling cells. At least 10,000 events were collected for each group.
Similar articles
- Dysregulation of IL-15-mediated T-cell homeostasis in TGF-beta dominant-negative receptor transgenic mice.
Lucas PJ, Kim SJ, Mackall CL, Telford WG, Chu YW, Hakim FT, Gress RE. Lucas PJ, et al. Blood. 2006 Oct 15;108(8):2789-95. doi: 10.1182/blood-2006-05-025676. Epub 2006 Jun 20. Blood. 2006. PMID: 16788095 Free PMC article. - Truncated form of TGF-βRII, but not its absence, induces memory CD8+ T cell expansion and lymphoproliferative disorder in mice.
Ishigame H, Mosaheb MM, Sanjabi S, Flavell RA. Ishigame H, et al. J Immunol. 2013 Jun 15;190(12):6340-50. doi: 10.4049/jimmunol.1300397. Epub 2013 May 17. J Immunol. 2013. PMID: 23686479 Free PMC article. - TGF-β sensitivity restrains CD8+ T cell homeostatic proliferation by enforcing sensitivity to IL-7 and IL-15.
Johnson LD, Jameson SC. Johnson LD, et al. PLoS One. 2012;7(8):e42268. doi: 10.1371/journal.pone.0042268. Epub 2012 Aug 6. PLoS One. 2012. PMID: 22879925 Free PMC article. - TGF-beta regulates pathology but not tissue CD8+ T cell dysfunction during experimental Trypanosoma cruzi infection.
Martin DL, Postan M, Lucas P, Gress R, Tarleton RL. Martin DL, et al. Eur J Immunol. 2007 Oct;37(10):2764-71. doi: 10.1002/eji.200737033. Eur J Immunol. 2007. PMID: 17823982 - TGF-beta: a master of all T cell trades.
Li MO, Flavell RA. Li MO, et al. Cell. 2008 Aug 8;134(3):392-404. doi: 10.1016/j.cell.2008.07.025. Cell. 2008. PMID: 18692464 Free PMC article. Review.
Cited by
- TGF-β broadly modifies rather than specifically suppresses reactivated memory CD8 T cells in a dose-dependent manner.
Taber A, Konecny A, Oda SK, Scott-Browne J, Prlic M. Taber A, et al. Proc Natl Acad Sci U S A. 2023 Nov 28;120(48):e2313228120. doi: 10.1073/pnas.2313228120. Epub 2023 Nov 21. Proc Natl Acad Sci U S A. 2023. PMID: 37988468 Free PMC article. - Depleting CD103+ resident memory T cells in vivo reveals immunostimulatory functions in oral mucosa.
Stolley JM, Scott MC, Joag V, Dale AJ, Johnston TS, Saavedra F, Gavil NV, Lotfi-Emran S, Soerens AG, Weyu E, Pierson MJ, Herzberg MC, Zhang N, Vezys V, Masopust D. Stolley JM, et al. J Exp Med. 2023 Jul 3;220(7):e20221853. doi: 10.1084/jem.20221853. Epub 2023 Apr 25. J Exp Med. 2023. PMID: 37097449 Free PMC article. - Shaping Heterogeneity of Naive CD8+ T Cell Pools.
Lee SW, Lee GW, Kim HO, Cho JH. Lee SW, et al. Immune Netw. 2023 Feb 22;23(1):e2. doi: 10.4110/in.2023.23.e2. eCollection 2023 Feb. Immune Netw. 2023. PMID: 36911807 Free PMC article. Review. - CAR Based Immunotherapy of Solid Tumours-A Clinically Based Review of Target Antigens.
Maher J, Davies DM. Maher J, et al. Biology (Basel). 2023 Feb 10;12(2):287. doi: 10.3390/biology12020287. Biology (Basel). 2023. PMID: 36829563 Free PMC article. Review. - Cytokines as an important player in the context of CAR-T cell therapy for cancer: Their role in tumor immunomodulation, manufacture, and clinical implications.
Silveira CRF, Corveloni AC, Caruso SR, Macêdo NA, Brussolo NM, Haddad F, Fernandes TR, de Andrade PV, Orellana MD, Guerino-Cunha RL. Silveira CRF, et al. Front Immunol. 2022 Sep 12;13:947648. doi: 10.3389/fimmu.2022.947648. eCollection 2022. Front Immunol. 2022. PMID: 36172343 Free PMC article. Review.
References
- Kingsley D.M. The TGF-β superfamilynew members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133–146. - PubMed
- Letterio J.J., Roberts A.B. Transforming growth factor-beta1-deficient miceidentification of isoform-specific activities in vivo. J. Leukoc. Biol. 1996;59:769–774. - PubMed
- Ruscetti F., Varesio L., Ochoa A., Ortaldo J. Pleiotropic effects of transforming growth factor-beta on cells of the immune system. Ann. NY Acad. Sci. 1993;685:488–500. - PubMed
- Gamble J.R., Khew-Goodall Y., Vadas M.A. Transforming growth factor-beta inhibits E-selectin expression on human endothelial cells. J. Immunol. 1993;150:4494–4503. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous