Lymph node trafficking and antigen presentation by endobronchial eosinophils - PubMed (original) (raw)
Lymph node trafficking and antigen presentation by endobronchial eosinophils
H Z Shi et al. J Clin Invest. 2000 Apr.
Abstract
Because eosinophils recruited into the airways in allergic diseases are exposed to inhaled allergens, we evaluated whether eosinophils within the endobronchial lumen can function in vivo as antigen-presenting cells for inhaled antigens. We recovered eosinophils from the airways after aerosol antigen challenge in sensitized mice or from the peritoneal cavities of IL-5 transgenic mice and fluorescently labeled these cells ex vivo. These labeled cells, instilled intratracheally into normal mice, migrated into draining paratracheal lymph nodes and localized to T cell-rich paracortical areas. The homing of airway eosinophils to lymph nodes was not governed by eotaxin, because CCR3(-/-) and CCR3(+/+) eosinophils migrated identically. Airway eosinophils, recovered after inhalational antigen challenge in sensitized mice, expressed MHC class II and costimulatory CD80 and CD86 proteins and functioned in vitro as CD80- and CD86-dependent, antigen-specific, antigen-presenting cells. Moreover, when instilled into the airways of antigen-sensitized recipient mice, airway eosinophils recovered after inhalational antigen challenge stimulated antigen-specific CD4(+) T cell proliferation within paratracheal lymph nodes. Thus, eosinophils within the lumina of airways can process inhaled antigens, traffic to regional lymph nodes, and function in vivo as antigen-presenting cells to stimulate responses of CD4(+) T cells.
Figures
Figure 1
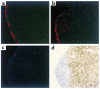
Eosinophil migration from the airways into paratracheal lymph nodes. Eosinophils (5 × 105) from the airways of antigen-sensitized and aerosol-challenged mice and labeled with DiIC16(3) (red), were instilled into the tracheas of recipient mice. Paratracheal lymph nodes were harvested at the indicated times after eosinophil instillation. Cryosectioned lymph nodes were stained with HOECHST 33342 (blue) to highlight nuclei and examined by fluorescence microscopy. T cell–rich regions were identified by immunoperoxidase staining with anti-CD3 mAb (brown). Labeled eosinophils were visible in the marginal sinus and the subcapsular region at 8 hours after instillation (a) and started to stream through the subcapsular sinus moving into the paracortical area at 16 hours (b). By 24 hours labeled eosinophils were predominantly located in the T-cell area, whereas few eosinophils were located in B-cell areas and in the subcapsular sinus (c, d). c and d are images of the same section for comparison. (Originally ×250.)
Figure 2
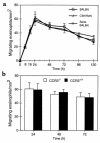
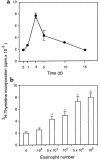
Eosinophil localization within paratracheal lymph nodes. After DiIC16(3)-labeled eosinophils (5 × 105) were instilled into the tracheas of recipient mice, paratracheal lymph nodes were examined by fluorescence microscopy to enumerate migrated eosinophils per square millimeter, as described in Methods. (a) Kinetics of eosinophil appearance in lymph nodes for normal BALB/c mice receiving airway eosinophils isolated from OVA-sensitized and aerosol-challenged BALB/c mice (G), for normal C3H/HeN mice receiving peritoneal eosinophils isolated from IL-5 C3H/HeN transgenic mice (J), and for OVA-sensitized BALB/c mice receiving airway eosinophils isolated from OVA-sensitized and aerosol-challenged BALB/c mice (H). Each result represents the mean ± SEM from 6 mice. (b) Comparisons of the lymph node migration of _CCR3_–/– and CCR3+/+ airway-derived eosinophils isolated from OVA-sensitized and aerosol-challenged mice instilled intratracheally into normal BALB/c mice. Each result represents the mean ± SEM from 3 mice per group.
Figure 3
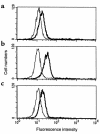
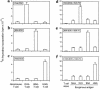
Expression of MHC class II molecules, CD80 and CD86, on airway eosinophils. Eosinophils, from BAL of BALB/c mice sensitized and aerosol-challenged with antigen, were stained with FITC-conjugated anti-I-Ad (a), anti-CD80 (b), or anti-CD86 (c) mAbs (thick lines) and analyzed by flow cytometry in comparison with isotype control FITC-conjugated IgG (thin lines). Results show a representative experiment from mice sensitized and challenged with OVA. Similar results were found with mice sensitized and aerosol challenged with BSA or HGG.
Figure 4
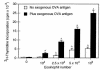
In vitro stimulation of sensitized T-lymphocyte proliferation by antigen-exposed and antigen-elicited airway eosinophils. OVA-sensitized T cells (2 × 105) were incubated with different numbers of airway eosinophils purified from OVA-sensitized and OVA-challenged mice in the absence and presence of 200 μg/mL exogenous OVA antigen. After 72 hours, cultures were pulsed with 3H-thymidine, and 3H-thymidine incorporation was determined 16–18 hours later. Results are mean 3H-thymidine incorporation ± SD from triplicate cultures. Data from a single experiment are representative of similar findings from 3 other experiments. A_P_ < 0.05, in comparison with values for no eosinophils, by ANOVA.
Figure 5
Antigen specificity of in vitro T-cell proliferation induced by antigen-exposed and antigen-elicited airway eosinophils. OVA-exposed (a), BSA-exposed (b), and HGG-exposed (c) airway eosinophils (EOS) were cocultured with nonimmune or OVA-, BSA-, or HGG-sensitized T cells without exogenous antigen. OVA-sensitized (d), BSA-sensitized (e), and HGG-sensitized (f) T cells (TC) were cocultured without eosinophils and exogenous antigen or with eosinophils purified from the airways of OVA-challenged (d), BSA-challenged (e), or HGG-challenged (f) mice with or with 200 μg/mL of exogenous OVA, BSA, or HGG. After 72 hours, cultures of 5 × 104 eosinophils and 2 × 105 T cells were pulsed with 3H-thymidine, and 3H-thymidine incorporation was determined 16–18 hours later. Results are mean 3H-thymidine incorporation ± SD from triplicate cultures. The data from a single experiment are representative of similar findings from 3 other experiments.
Figure 6
Kinetics and dose dependence of in vivo T-cell proliferation elicited by antigen-exposed and antigen-elicited airway eosinophils. (a) OVA-elicited airway eosinophils (5 × 105) were instilled into the tracheas of OVA-sensitized mice, and paratracheal lymph nodes were taken at indicated times and evaluated for in vivo–induced proliferation. (b) Increasing numbers (104–106) of OVA-elicited airway eosinophils were instilled into the tracheas of OVA-immunized mice, and paratracheal lymph nodes, taken 3 days after instillation, were evaluated for in vivo–induced proliferation. In each experiment 3 × 105 lymph node cells were pulsed immediately with 3H-thymidine, incubated for 18 hours, and assessed for 3H-thymidine incorporation. Results are the mean 3H-thymidine incorporation ± SEM of 3 independent experiments. A_P_ < 0.01, compared with controls in which no eosinophils were used, by ANOVA.
Figure 7
The antigen specificity of in vivo T-cell proliferation elicited by antigen-exposed and antigen-elicited airway eosinophils. OVA-elicited (a), BSA-elicited (b), and HGG-elicited (c) airway eosinophils (5 × 105) were instilled into the tracheas of nonimmune or OVA-, BSA-, or HGG-immunized mice. Paratracheal lymph nodes, taken 3 days after instillation, were evaluated for in vivo–induced proliferation. Lymph node cells (3 × 105) were pulsed with 3H-thymidine, incubated for 16–18 hours, and assessed for 3H-thymidine incorporation. Results are the mean 3H-thymidine incorporation ± SD of triplicate cultures. The data with a single experiment are representative of similar findings with 3 other experiments.
Figure 8
CD4+ but not CD8+ T cells respond in vivo to antigen-presenting eosinophils. PBS (a, c) or antigen-exposed eosinophils (b, d) were instilled into the tracheas of antigen-sensitized mice. As described in Methods, mice were administered BrdU, and T cells were purified from lymph nodes 3 days after eosinophil instillation, double-stained with FITC–anti-BrdU mAb and PE–anti-CD4 (a, b) or anti-CD8 mAb (c, d) and analyzed by flow cytometry. Results show 1 experiment representative of 5 independent experiments from OVA-immunized mice receiving OVA-exposed eosinophils; similar results were also found in BSA-immunized mice receiving BSA-exposed eosinophils and HGG-immunized mice receiving HGG-exposed eosinophils. Numbers specify the percentage of cells in each of the 4 quadrants.
Similar articles
- Endobronchial eosinophils preferentially stimulate T helper cell type 2 responses.
Shi HZ, Xiao CQ, Li CQ, Mo XY, Yang QL, Leng J, Chen YQ. Shi HZ, et al. Allergy. 2004 Apr;59(4):428-35. doi: 10.1046/j.1398-9995.2003.00405.x. Allergy. 2004. PMID: 15005767 - [Endobronchial eosinophils preferentially stimulate T helper cell type 2 responses].
Shi HZ, Yang QL, Mo XY, Chen YQ, Leng J, Deng JM. Shi HZ, et al. Zhonghua Yi Xue Za Zhi. 2003 Dec 25;83(24):2162-5. Zhonghua Yi Xue Za Zhi. 2003. PMID: 14720427 Chinese. - Effects of antigen presentation of eosinophils on lung Th1/Th2 imbalance.
Xie ZF, Shi HZ, Qin XJ, Kang LF, Huang CP, Chen YQ. Xie ZF, et al. Chin Med J (Engl). 2005 Jan 5;118(1):6-11. Chin Med J (Engl). 2005. PMID: 15642219 - Eosinophils function as antigen-presenting cells.
Shi HZ. Shi HZ. J Leukoc Biol. 2004 Sep;76(3):520-7. doi: 10.1189/jlb.0404228. Epub 2004 Jun 24. J Leukoc Biol. 2004. PMID: 15218055 Review. - Antigen presentation to naive CD4 T cells in the lymph node.
Itano AA, Jenkins MK. Itano AA, et al. Nat Immunol. 2003 Aug;4(8):733-9. doi: 10.1038/ni957. Nat Immunol. 2003. PMID: 12888794 Review.
Cited by
- Allergy, asthma, and inflammation: which inflammatory cell type is more important?
Moqbel R, Odemuyiwa SO. Moqbel R, et al. Allergy Asthma Clin Immunol. 2008 Dec 15;4(4):150-6. doi: 10.1186/1710-1492-4-4-150. Epub 2008 Dec 15. Allergy Asthma Clin Immunol. 2008. PMID: 20525138 Free PMC article. - Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen.
Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Hammad H, et al. J Exp Med. 2010 Sep 27;207(10):2097-111. doi: 10.1084/jem.20101563. Epub 2010 Sep 6. J Exp Med. 2010. PMID: 20819925 Free PMC article. - Accumulation of eosinophils in intestine-draining mesenteric lymph nodes occurs after Trichuris muris infection.
Svensson M, Bell L, Little MC, DeSchoolmeester M, Locksley RM, Else KJ. Svensson M, et al. Parasite Immunol. 2011 Jan;33(1):1-11. doi: 10.1111/j.1365-3024.2010.01246.x. Parasite Immunol. 2011. PMID: 21155838 Free PMC article. - Eosinophils in the pathogenesis of pancreatic disorders.
Manohar M, Kandikattu HK, Upparahalli Venkateshaiah S, Yadavalli CS, Mishra A. Manohar M, et al. Semin Immunopathol. 2021 Jun;43(3):411-422. doi: 10.1007/s00281-021-00853-0. Epub 2021 Mar 30. Semin Immunopathol. 2021. PMID: 33783592 Free PMC article. Review. - Cellular and molecular mediators of lymphangiogenesis in inflammatory bowel disease.
Ocansey DKW, Pei B, Xu X, Zhang L, Olovo CV, Mao F. Ocansey DKW, et al. J Transl Med. 2021 Jun 10;19(1):254. doi: 10.1186/s12967-021-02922-2. J Transl Med. 2021. PMID: 34112196 Free PMC article. Review.
References
- Seminario MC, Gleich GJ. The role of eosinophils in the pathogenesis of asthma. Curr Opin Immunol. 1994;6:860–864. - PubMed
- Bentley AM, et al. Increases in activated T lymphocytes, eosinophils, and cytokine mRNA expression for interleukin-5 and granulocyte/macrophage colony-stimulating factor in bronchial biopsies after allergen challenge in atopic asthmatics. Am J Respir Cell Mol Biol. 1993;8:35–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI22571/AI/NIAID NIH HHS/United States
- HL46563/HL/NHLBI NIH HHS/United States
- R01 AI020241/AI/NIAID NIH HHS/United States
- R01 AI022571/AI/NIAID NIH HHS/United States
- AI20241/AI/NIAID NIH HHS/United States
- R37 AI020241/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials