Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons - PubMed (original) (raw)
Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons
C Kaether et al. Mol Biol Cell. 2000 Apr.
Free PMC article
Abstract
Neurons transport newly synthesized membrane proteins along axons by microtubule-mediated fast axonal transport. Membrane proteins destined for different axonal subdomains are thought to be transported in different transport carriers. To analyze this differential transport in living neurons, we tagged the amyloid precursor protein (APP) and synaptophysin (p38) with green fluorescent protein (GFP) variants. The resulting fusion proteins, APP-yellow fluorescent protein (YFP), p38-enhanced GFP, and p38-enhanced cyan fluorescent protein, were expressed in hippocampal neurons, and the cells were imaged by video microscopy. APP-YFP was transported in elongated tubules that moved extremely fast (on average 4.5 micrometer/s) and over long distances. In contrast, p38-enhanced GFP-transporting structures were more vesicular and moved four times slower (0.9 micrometer/s) and over shorter distances only. Two-color video microscopy showed that the two proteins were sorted to different carriers that moved with different characteristics along axons of doubly transfected neurons. Antisense treatment using oligonucleotides against the kinesin heavy chain slowed down the long, continuous movement of APP-YFP tubules and increased frequency of directional changes. These results demonstrate for the first time directly the sorting and transport of two axonal membrane proteins into different carriers. Moreover, the extremely fast-moving tubules represent a previously unidentified type of axonal carrier.
Figures
Figure 1
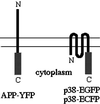
Schematic diagram of GFP fusion proteins.YFP was fused to the C terminus of human APP695 (left). EGFP and ECFP were fused to the C terminus of rat p38 (right). In both constructs the FP is in the cytoplasm. The double line represents the plasma membrane.
Figure 2
Transfected GFP fusion proteins are expressed as full-length proteins. (A) Cell lysates (C) and media (M) of 8-d-old APP-YFP–transfected (+) and mock-transfected (−) neurons were analyzed by SDS-PAGE and immunoblotting with GFP antiserum (αGFP). The asterisk marks a nonspecific band visible in transfected and control neurons; the arrow points to a minor degradation band. After stripping, the blot was reprobed with an antibody against APP (αAPP). Full-length APP-YFP as well as endogenous rat APP were detected. Molecular mass markers are indicated in kilodaltons (B) Cell lysates of 9-d-old p38-EGFP–transfected (+) and mock-transfected (−) neurons were analyzed by SDS-PAGE and immunoblotting with GFP antiserum D2 (αGFP). The asterisk marks a prominent nonspecific band. Molecular mass markers are indicated in kilodaltons.
Figure 3
GFP-labeled fusion proteins are sorted to axons. Seven- to 10-d-old neurons were transfected with APP-YFP (A and B) or p38-EGFP (C and D) cDNA, fixed after 1 d, and processed for immunofluorescence using anti-GFP antiserum (αGFP) and anti-MAP2 monoclonal antibody (αMAP2). Arrows indicate axons as defined by the absence of MAP2 labeling. Bar, 10 μm.
Figure 4
APP-YFP is transported in long tubules along axons (video). Nine-day-old neurons were transfected with APP-YFP cDNA and analyzed 24 h later by video microscopy. Frames were taken every 0.9 s; 6 subsequent frames of 50 frames (total length, 45 s) are shown. Arrows depict fast anterogradely moving, APP-YFP–containing tubules; arrowheads indicate retrogradely moving tubules. The asterisk points to nonmoving, vesicular structures. Bar. 10 μm.
Figure 5
p38-EGFP is transported in tubulovesicular structures (video). Seven- to 8-d-old neurons were transfected with p38-EGFP cDNA and analyzed 24 h later by video microscopy. Frames were taken every 3 s; two subsequent frames of 70 (total length, 210 s) are shown. White arrows depict moving, p38-EGFP–containing tubules; gray arrows indicate the positions of the structures in the previous frame. Arrowheads point to nonmoving structures. Top, anterograde. Bar, 10 μm.
Figure 6
Moving p38-EGFP–containing vesicles do not contain an endosomal marker. Eight-day-old neurons were transfected with p38-EGFP cDNA, labeled 22 h later for 1–2 h with 1 mg/ml Rh-dextran, and analyzed by two-color video microscopy as described in MATERIALS AND METHODS. Top, consecutive frames showing p38-EGFP-fluorescence; bottom, corresponding frames showing Rh-dextran fluorescence. Arrowheads point to moving p38-EGFP–containing vesicles; arrows point to a moving Rh-dextran–containing vesicle. Asterisks mark nonmoving structures that contain both markes. Note that moving vesicles contain one marker only. Bar, 10 μm.
Figure 7
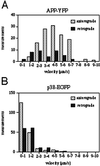
Velocity profile of axonal transport of APP-YFP in comparison with p38-EGFP. Neurons were transfected with APP-YFP and p38-EGFP cDNA, respectively, and analyzed by video microscopy. Moving structures were tracked, and the velocity was calculated as described in MATERIALS AND METHODS. (A) Velocity profile of APP-YFP–containing moving structures. (B) Velocity profile of p38-EGFP–containing moving structures. Measurements, number of measured velocities between two frames.
Figure 8
Two-color video microscopy of doubly transfected neurons shows little colocalization of APP-YFP and p38-ECFP (video). Seven- to 8-d-old neurons were doubly transfected with APP-YFP and p38-ECFP cDNA, and two-color video microscopy was performed as described in MATERIALS AND METHODS. (A), Left, frames showing APP-YFP (video); middle, frames showing p38-EGFP (video); right, merged images (video). APP-YFP is shown in green; p38-ECFP is shown in red. (B) Five subsequent frames from the boxed area in A are displayed separately. Top row, APP-YFP; bottom row, p38-ECFP. Arrows mark the position of a fast-moving APP-YFP–positive, p38-ECFP–negative tubule; arrowheads mark the position of a retrogradely moving vesicular structure containing both proteins. The triangle marks the position of a p38-ECFP–positive, APP-YFP–negative structure moving to the right of the image. The total number of frames is 20 pairs, recorded during 64 s. Bar, 10 μm.
Figure 9
KHC antisense treatment interferes with axonal transport of APP-YFP (video). Seven- to 8-d-old neurons were transfected with APP-YFP and incubated overnight with sense (s) and antisense (as) oligonucleotides corresponding to KHC. Video microscopy was performed, and tracks were analyzed. Every second frame of eight frames and the corresponding track displays are shown for both conditions. Top, anterograde. Total length: sense, 34 frames recorded in 44 s; antisense, 71 frames recorded in 123 s. Bar, 10 μm.
Similar articles
- The beta-amyloid domain is essential for axonal sorting of amyloid precursor protein.
Tienari PJ, De Strooper B, Ikonen E, Simons M, Weidemann A, Czech C, Hartmann T, Ida N, Multhaup G, Masters CL, Van Leuven F, Beyreuther K, Dotti CG. Tienari PJ, et al. EMBO J. 1996 Oct 1;15(19):5218-29. EMBO J. 1996. PMID: 8895567 Free PMC article. - Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head.
Nakata T, Hirokawa N. Nakata T, et al. J Cell Biol. 2003 Sep 15;162(6):1045-55. doi: 10.1083/jcb.200302175. J Cell Biol. 2003. PMID: 12975348 Free PMC article. - Rapid movement of axonal neurofilaments interrupted by prolonged pauses.
Wang L, Ho CL, Sun D, Liem RK, Brown A. Wang L, et al. Nat Cell Biol. 2000 Mar;2(3):137-41. doi: 10.1038/35004008. Nat Cell Biol. 2000. PMID: 10707083 - Subcellular trafficking of the amyloid precursor protein gene family and its pathogenic role in Alzheimer's disease.
Kins S, Lauther N, Szodorai A, Beyreuther K. Kins S, et al. Neurodegener Dis. 2006;3(4-5):218-26. doi: 10.1159/000095259. Neurodegener Dis. 2006. PMID: 17047360 Review. - Membrane traffic in polarized neurons in culture.
de Hoop MJ, Dotti CG. de Hoop MJ, et al. J Cell Sci Suppl. 1993;17:85-92. doi: 10.1242/jcs.1993.supplement_17.13. J Cell Sci Suppl. 1993. PMID: 8144707 Review.
Cited by
- Cellular Processes Induced by HSV-1 Infections in Vestibular Neuritis.
Zhao Z, Liu X, Zong Y, Shi X, Sun Y. Zhao Z, et al. Viruses. 2023 Dec 20;16(1):12. doi: 10.3390/v16010012. Viruses. 2023. PMID: 38275947 Free PMC article. Review. - Hook proteins: association with Alzheimer pathology and regulatory role of hook3 in amyloid beta generation.
Herrmann L, Wiegmann C, Arsalan-Werner A, Hilbrich I, Jäger C, Flach K, Suttkus A, Lachmann I, Arendt T, Holzer M. Herrmann L, et al. PLoS One. 2015 Mar 23;10(3):e0119423. doi: 10.1371/journal.pone.0119423. eCollection 2015. PLoS One. 2015. PMID: 25799409 Free PMC article. - Regulators of kinesin involved in polarized trafficking and axon outgrowth.
Luo S, Nonet ML. Luo S, et al. J Biol. 2006;5(4):8. doi: 10.1186/jbiol41. Epub 2006 May 25. J Biol. 2006. PMID: 16732897 Free PMC article. - Calsyntenin-1 docks vesicular cargo to kinesin-1.
Konecna A, Frischknecht R, Kinter J, Ludwig A, Steuble M, Meskenaite V, Indermühle M, Engel M, Cen C, Mateos JM, Streit P, Sonderegger P. Konecna A, et al. Mol Biol Cell. 2006 Aug;17(8):3651-63. doi: 10.1091/mbc.e06-02-0112. Epub 2006 Jun 7. Mol Biol Cell. 2006. PMID: 16760430 Free PMC article. - Kinesin light chain-1 serine-460 phosphorylation is altered in Alzheimer's disease and regulates axonal transport and processing of the amyloid precursor protein.
Mórotz GM, Glennon EB, Greig J, Lau DHW, Bhembre N, Mattedi F, Muschalik N, Noble W, Vagnoni A, Miller CCJ. Mórotz GM, et al. Acta Neuropathol Commun. 2019 Dec 5;7(1):200. doi: 10.1186/s40478-019-0857-5. Acta Neuropathol Commun. 2019. PMID: 31806024 Free PMC article.
References
- Allen RD, Metuzals J, Tasaki I, Brady ST, Gilbert SP. Fast axonal transport in squid giant axon. Science. 1982;218:1127–1129. - PubMed
- Amaratunga A, Morin PJ, Kosik KS, Fine RE. Inhibition of kinesin synthesis and rapid anterograde axonal transport in vivo by an antisense oligonucleotide. J Biol Chem. 1993;268:17427–17430. - PubMed
- Bradke F, Dotti CG. Neuronal polarity: vectorial cytoplasmic flow precedes axon formation. Neuron. 1997;19:1175–1186. - PubMed
- Brady ST. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985;317:73–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources