The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice - PubMed (original) (raw)
The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice
G E Price et al. J Virol. 2000 May.
Abstract
During influenza virus infection innate and adaptive immune defenses are activated to eliminate the virus and thereby bring about recovery from illness. Both arms of the adaptive immune system, antibody neutralization of free virus and termination of intracellular virus replication by antiviral cytotoxic T cells (CTLs), play pivotal roles in virus elimination and protection from disease. Innate cytokine responses, such as alpha/beta interferon (IFN-alpha/beta) or IFN-gamma, can have roles in determining the rate of virus replication in the initial stages of infection and in shaping the initial inflammatory and downstream adaptive immune responses. The effect of these cytokines on the replication of pneumotropic influenza A virus in the respiratory tract and in the regulation of adaptive antiviral immunity was examined after intranasal infection of mice with null mutations in receptors for IFN-alpha/beta, IFN-gamma, and both IFNs. Virus titers in the lungs of mice unable to respond to IFNs were not significantly different from congenic controls for both primary and secondary infection. Likewise the mice were comparably susceptible to X31 (H3N2) influenza virus infection. No significant disruption to the development of normal antiviral CTL or antibody responses was observed. In contrast, mice bearing the disrupted IFN-alpha/beta receptor exhibited accelerated kinetics and significantly higher levels of neutralizing antibody activity during primary or secondary heterosubtypic influenza virus infection. Thus, these observations reveal no significant contribution for IFN-controlled pathways in shaping acute or memory T-cell responses to pneumotropic influenza virus infection but do indicate some role for IFN-alpha/beta in the regulation of antibody responses. Recognizing the pivotal role of CTLs and antibody in virus clearance, it is reasonable to assume a redundancy in IFN-mediated antiviral effects in pulmonary influenza. However, IFN-alpha/beta seems to be a valid factor in determining tissue tropism and replicative rates of highly virulent influenza virus strains as reported previously by others, and this aspect is discussed here.
Figures
FIG. 1
Susceptibility to influenza virus infection of mice lacking receptors for IFN-α/β, IFN-γ, or both IFNs. 129/SvEv, IFNα/βR−/−, IFNγR−/−, and IFNα/β-γR−/− mice were infected with X31, and the survival of infected mice was observed over a period of 25 days. The percent survival is shown for groups of 10 to 15 mice. Virus was administered i.n. at doses of 107 PFU (●), 106 PFU (▴), 105 PFU (▾), 104 PFU (⧫) or 102 PFU (■).
FIG. 2
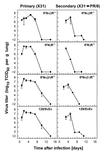
Kinetics of lung virus replication after primary (X31) or challenge infection with A/PR/8/34 influenza A virus in mice lacking receptors for IFN-α/β, IFN-γ, or both IFNs compared to their 129/SvEv congenic controls. Lung virus titers were measured following infection of naive (primary) IFNα/βR−/−, IFNγR−/−, IFNα/β-γR−/−, or 129/SvEv control mice with 500 PFU of X31 (left panels) or following challenge of mice which had been primed with 500 PFU of X31 30 days previously with the heterologous A/PR/8/34 influenza (500 PFU i.n.) (right panels). Lung virus titers are expressed as the mean ± the standard error of the mean (SEM) log10 TCID50/gram of lung tissue of three to five mice.
FIG. 3
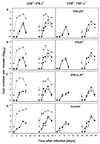
Virus-specific CD8+ T cells in primary and challenge influenza infection of mice lacking receptors for IFN-α/β, IFN-γ, or both IFNs compared to controls. Naive IFNα/βR−/− (A), IFNγR−/− (B), IFNα/β-γR−/− (C), or control (D) mice were infected with 500 PFU of X31, and the numbers of virus-specific CD8+ T cells in the BAL fluid were measured. BAL samples from each group of three to five mice were pooled, and the numbers of virus-specific CTLs were determined by staining CD8+ T cells for intracellular IFN-γ (left panels) or TNF-α (right panels) secretion, following stimulation of cells with NP366–374 (●) or NS2114–121 (▴) viral peptide. Alternatively, X31 primed mice (500 PFU i.n.) were challenged with A/PR/8/34 (500 PFU i.n.) 30 days later (as indicated by the arrow), and the numbers of virus-specific CD8+ T cells were determined as described above. The BAL cell counts per mouse (◊) were used, together with the flow cytometry data, to calculate the average numbers for the total CD8+ T cells specific to NP366–374 or NS2114–121 peptide epitope. BAL samples (total volume, 1 ml/lung) containing <104 cells/ml (the limit of detection of our hemocytometer counting assay) were estimated as 104 cells per lung.
FIG. 4
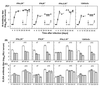
Generation and maintenance of primary or memory virus-specific antibody responses of mice lacking receptors for IFN-α/β, IFN-γ, or both IFNs compared to controls. (A) The ability of IFNα/βR−/−, IFNγR−/−, IFNα/β-γR−/−, or 129/SvEv control mice to produce protective neutralizing antibodies was tested by measuring HI antibody titers in the sera of mice infected i.n. with 500 PFU of X31 (H3N2) (primary infection), or following their challenge on day 30 after primary infection (as indicated by the arrow), with 500 PFU of the heterologous A/PR/8/34 (H1N1) virus. The titers of X31 (●)- and A/PR/8/34 (○)-specific HI antibodies were estimated individually, and the results are expressed as the mean ± the SEM log10 HI antibody titers of groups of three to five mice. The isotype pattern of antibodies in the sera of IFNα/βR−/−, IFNγR−/−, IFNα/β-γR−/−, or 129/SvEv control mice following i.n. infection with 500 PFU of X31 was measured on days 7 and 10 after virus inoculation. (B and C) The results are shown as an ELISA titer of virus-specific antibody (mean ± the SEM log10 of three to five mice) of the IgM, IgG, or IgA isotype (B) or the IgG1, IgG2a, IgG2b, or IgG3 isotype (C).
Similar articles
- Gamma interferon is not required for mucosal cytotoxic T-lymphocyte responses or heterosubtypic immunity to influenza A virus infection in mice.
Nguyen HH, van Ginkel FW, Vu HL, Novak MJ, McGhee JR, Mestecky J. Nguyen HH, et al. J Virol. 2000 Jun;74(12):5495-501. doi: 10.1128/jvi.74.12.5495-5501.2000. J Virol. 2000. PMID: 10823854 Free PMC article. - Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells.
Ou R, Zhou S, Huang L, Moskophidis D. Ou R, et al. J Virol. 2001 Sep;75(18):8407-23. doi: 10.1128/jvi.75.18.8407-8423.2001. J Virol. 2001. PMID: 11507186 Free PMC article. - Protective role of gamma interferon during the recall response to influenza virus.
Bot A, Bot S, Bona CA. Bot A, et al. J Virol. 1998 Aug;72(8):6637-45. doi: 10.1128/JVI.72.8.6637-6645.1998. J Virol. 1998. PMID: 9658110 Free PMC article. - Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense.
Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, Puel A, Picard C, Abel L, Jouanguy E, Casanova JL. Zhang SY, et al. Immunol Rev. 2008 Dec;226:29-40. doi: 10.1111/j.1600-065X.2008.00698.x. Immunol Rev. 2008. PMID: 19161414 Review. - The Role of Type I IFNs in Influenza: Antiviral Superheroes or Immunopathogenic Villains?
Wu W, Metcalf JP. Wu W, et al. J Innate Immun. 2020;12(6):437-447. doi: 10.1159/000508379. Epub 2020 Jun 19. J Innate Immun. 2020. PMID: 32564033 Free PMC article. Review.
Cited by
- Consecutive BNT162b2 mRNA vaccination induces short-term epigenetic memory in innate immune cells.
Yamaguchi Y, Kato Y, Edahiro R, Søndergaard JN, Murakami T, Amiya S, Nameki S, Yoshimine Y, Morita T, Takeshima Y, Sakakibara S, Naito Y, Motooka D, Liu YC, Shirai Y, Okita Y, Fujimoto J, Hirata H, Takeda Y, Wing JB, Okuzaki D, Okada Y, Kumanogoh A. Yamaguchi Y, et al. JCI Insight. 2022 Nov 22;7(22):e163347. doi: 10.1172/jci.insight.163347. JCI Insight. 2022. PMID: 36282593 Free PMC article. - Oxygen free radical involvement in acute lung injury induced by H5N1 virus in mice.
He G, Dong C, Luan Z, McAllan BM, Xu T, Zhao L, Qiao J. He G, et al. Influenza Other Respir Viruses. 2013 Nov;7(6):945-53. doi: 10.1111/irv.12067. Epub 2013 Jan 22. Influenza Other Respir Viruses. 2013. PMID: 23336583 Free PMC article. - Interferon induction and function at the mucosal surface.
Durbin RK, Kotenko SV, Durbin JE. Durbin RK, et al. Immunol Rev. 2013 Sep;255(1):25-39. doi: 10.1111/imr.12101. Immunol Rev. 2013. PMID: 23947345 Free PMC article. Review. - Toll-like receptor-independent triggering of dendritic cell maturation by viruses.
López CB, Yount JS, Moran TM. López CB, et al. J Virol. 2006 Apr;80(7):3128-34. doi: 10.1128/JVI.80.7.3128-3134.2006. J Virol. 2006. PMID: 16537581 Free PMC article. Review. No abstract available. - Detection of mouse-adapted human influenza virus in the olfactory bulbs of mice within hours after intranasal infection.
Majde JA, Bohnet SG, Ellis GA, Churchill L, Leyva-Grado V, Wu M, Szentirmai E, Rehman A, Krueger JM. Majde JA, et al. J Neurovirol. 2007 Oct;13(5):399-409. doi: 10.1080/13550280701427069. J Neurovirol. 2007. PMID: 17994424
References
- Barrett T, Inglis S C. Growth, purification and titration of influenza viruses. In: Mahy B W J, editor. Virology: a practical approach. Oxford, England: IRL Press; 1985. pp. 119–150.
- Biron C A. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr Opin Immunol. 1994;6:530–538. - PubMed
- Biron C A. Initial and innate responses to viral infections—pattern setting in immunity or disease. Curr Opin Microbiol. 1999;2:374–381. - PubMed
- Biron C A. Role of early cytokines, including alpha and beta interferons (IFN-α/β), in innate and adaptive immune responses to viral infections. Semin Immunol. 1998;10:383–390. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases