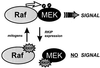Mechanism of suppression of the Raf/MEK/extracellular signal-regulated kinase pathway by the raf kinase inhibitor protein - PubMed (original) (raw)
Mechanism of suppression of the Raf/MEK/extracellular signal-regulated kinase pathway by the raf kinase inhibitor protein
K Yeung et al. Mol Cell Biol. 2000 May.
Abstract
We have recently identified the Raf kinase inhibitor protein (RKIP) as a physiological endogenous inhibitor of the Raf-1/MEK/extracellular signal-regulated kinase (ERK) pathway. RKIP interfered with MEK phosphorylation and activation by Raf-1, resulting in the suppression of both Raf-1-induced transformation and AP-1-dependent transcription. Here we report the molecular mechanism of RKIP's inhibitory function. RKIP can form ternary complexes with Raf-1, MEK, and ERK. However, whereas MEK and ERK can simultaneously associate with RKIP, Raf-1 binding to RKIP and that of MEK are mutually exclusive. RKIP is able to dissociate a Raf-1-MEK complex and behaves as a competitive inhibitor of MEK phosphorylation. Mapping of the binding domains showed that MEK and Raf-1 bind to overlapping sites in RKIP, whereas MEK and RKIP associate with different domains in Raf-1, and Raf-1 and RKIP bind to different sites in MEK. Both the Raf-1 and the MEK binding sites in RKIP need to be destroyed in order to relieve RKIP-mediated suppression of the Raf-1/MEK/ERK pathway, indicating that binding of either Raf-1 or MEK is sufficient for inhibition. The properties of RKIP reveal the specific sequestration of interacting components as a novel motif in the cell's repertoire for the regulation of signaling pathways.
Figures
FIG. 1
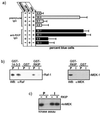
RKIP inhibits the ERK pathway by preventing MEK activation. (a) Rat-1 cells were microinjected with a TRE-LacZ reporter plasmid and affinity-purified RKIP antibodies or preimmune immunoglobulin G (IgG) and treated as indicated. The MEK inhibitors PD98059 and U0126 were administered 1 h before microinjection of TPA (100 ng/ml). (b) RKIP antibodies prevent binding of RKIP to Raf-1 or MEK. GST, GST-RKIP, or GST–14-3-3 beads were incubated with saturating amounts of RKIP antibodies (I) or the corresponding preimmune serum (P) and tested for binding of Raf-1 or MEK-1. WB, Western blot. (c) The phosphorylation of kinase-negative MEK-1 (knMEK) by activated Raf-1 was examined in the presence (+) or absence (−) of 10 μM purified RKIP. RKIP was preincubated with RKIP antibodies or the corresponding preimmune serum for 1 h.
FIG. 2
Analysis of the composition of RKIP protein complexes. (a) GST-MEK beads were incubated with RKIP, Raf, and MEK in the indicated combinations. GST-RKIP beads (b), GST-ERK beads (c), or GST-Raf-1 beads (d) were incubated with recombinant purified proteins as indicated. Incubations were done as described in Materials and Methods, and associated proteins were visualized by Western blotting.
FIG. 3
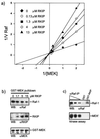
RKIP inhibits Raf-1 by a competitive mechanism. (a) Lineweaver-Burk plot of Raf-1 inhibition by RKIP. Activated GST–Raf-1 was used to phosphorylate GST–MEK-1 in the presence of increasing amounts of RKIP, as indicated. Phosphorylation was quantified with a Fuji phosphorimager. The data shown are the averages of three independent experiments. (b) RKIP disrupts the Raf-1–MEK complex. GST-MEK and Raf-1 were coexpressed in Sf-9 cells. The GST-MEK–Raf-1 complex was purified by adsorption to glutathione Sepharose beads, washed, and resuspended in PBS. Purified RKIP was added at the concentrations indicated. After 1 h at 4°C, the GST-MEK beads were washed three times with PBS and examined for associated proteins by Western blotting (WB) with the indicated antisera. (c) Raf-1 bound to RKIP does not phosphorylate MEK. A lysate of Sf-9 cells expressing activated Raf-1 was incubated with 5 μg of GST or GST-RKIP beads. Serial dilutions of the same lysate were immunoprecipitated with the anti-Raf serum crafVI. After three washes with PBS, the pellets were resuspended in kinase buffer and incubated with 100 μM ATP and kinase-negative MEK as substrate. MEK phosphorylation was visualized by immunoblotting with a phospho-MEK-specific antiserum. Raf-1 was stained with crafVI.
FIG. 4
Analysis of RKIP binding to activated Raf-1, MEK, and ERK. (a) Mitogen activation of Raf-1 decreases its association with RKIP. COS-1 cells were transiently transfected with Raf-1 and RKIP expression vectors. Serum-starved cells were treated with epidermal growth factor (EGF) (20 ng/ml) plus TPA (100 ng/ml) for the times indicated. Raf-1 immunoprecipitates were analyzed for kinase activity, and RKIP immunoprecipitates were examined for Raf-1. IP, immunoprecipitation; WB, Western blot. (b) Purified RKIP produced in E. coli was tested for binding to GST-Raf and activated (*) GST-Raf beads. GST-Raf proteins were produced in Sf-9 cells and activated by coexpression of RasV12 and Lck. An aliquot of the GST-Raf beads was examined for phosphorylation of kinase-negative MEK (knMEK). (c and d) MEK and ERK proteins were phosphorylated in the presence of [γ-32P]ATP and tested for binding to GST-RKIP beads. Binding of phosphorylated proteins was detected by autoradiography. Binding of total protein was visualized by Western blotting (WB). The contribution of phosphoproteins to the Western blot signal is minimal, because they represent less than 10% of the total protein.
FIG. 5
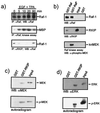
Analysis of binding domains. (a) RKIP and MEK bind to different domains of the Raf-1 kinase. GST-tagged BXB, GNX, and the indicated deletion mutants were expressed in E. coli, immobilized on glutathione Sepharose beads, and incubated with purified RKIP or MEK-1. Proteins were visualized by Western blotting. The diagram illustrates the GNX regions deduced to be required for binding. Roman numerals refer to the kinase subdomains as defined by Hanks and Quinn (8). (b) RKIP and Raf-1 bind to different domains of MEK-1. Purified six-His-tagged MEK-1 deletion mutants were tested for binding to GST-RKIP beads (left panel) and GST–Raf-1 beads (right panel). His/MEK-1 proteins were detected by Western blotting with anti-His antibodies. The lower panel shows a schematic summary. nd, not done. (c) Analysis of Raf-1 and MEK binding sites in RKIP. GST-RKIP deletion mutants were tested for binding of MEK-1 and Raf-1. PEB, phosphatidylethanolamine binding motif.
FIG. 6
RKIP binding to Raf-1 or MEK is sufficient for inhibition. (a) Coimmunoprecipitation of RKIP deletion mutants with Raf-1. FLAG–Raf-1 and hemagglutinin (HA)-RKIP or HA-RKIP deletion mutants were coexpressed in COS cells. Lysates were immunoprecipitated (IP) with anti-FLAG antibodies, and associated HA-RKIP proteins were detected by Western blotting (WB) with anti-HA antibodies. PEB, phosphatidylethanolamine binding motif. (b) The effect of RKIP deletion mutants on Raf-induced AP-1 reporter gene expression. HA-RKIP mutants were cotransfected with the Raf-1 kinase domain, BXB, and an AP-1–luciferase plasmid.
FIG. 7
Model of RKIP function. The activation of MEK by Raf-1 requires physical interaction between Raf-1 and MEK. RKIP binding to either Raf-1 or MEK dissociates Raf-MEK complexes and thereby interrupts MEK activation and downstream signaling. The binding of RKIP to Raf-1 is negatively regulated by mitogens.
Similar articles
- Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP.
Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, Sedivy JM, Kolch W. Yeung K, et al. Nature. 1999 Sep 9;401(6749):173-7. doi: 10.1038/43686. Nature. 1999. PMID: 10490027 - Raf kinase inhibitor protein interacts with NF-kappaB-inducing kinase and TAK1 and inhibits NF-kappaB activation.
Yeung KC, Rose DW, Dhillon AS, Yaros D, Gustafsson M, Chatterjee D, McFerran B, Wyche J, Kolch W, Sedivy JM. Yeung KC, et al. Mol Cell Biol. 2001 Nov;21(21):7207-17. doi: 10.1128/MCB.21.21.7207-7217.2001. Mol Cell Biol. 2001. PMID: 11585904 Free PMC article. - The RKIP (Raf-1 Kinase Inhibitor Protein) conserved pocket binds to the phosphorylated N-region of Raf-1 and inhibits the Raf-1-mediated activated phosphorylation of MEK.
Rath O, Park S, Tang HH, Banfield MJ, Brady RL, Lee YC, Dignam JD, Sedivy JM, Kolch W, Yeung KC. Rath O, et al. Cell Signal. 2008 May;20(5):935-41. doi: 10.1016/j.cellsig.2008.01.012. Epub 2008 Jan 24. Cell Signal. 2008. PMID: 18294816 - The role of Raf kinase inhibitor protein (RKIP) in health and disease.
Keller ET, Fu Z, Brennan M. Keller ET, et al. Biochem Pharmacol. 2004 Sep 15;68(6):1049-53. doi: 10.1016/j.bcp.2004.04.024. Biochem Pharmacol. 2004. PMID: 15313400 Review. - Regulation of the MAPK pathway by raf kinase inhibitory protein.
Vandamme D, Herrero A, Al-Mulla F, Kolch W. Vandamme D, et al. Crit Rev Oncog. 2014;19(6):405-15. doi: 10.1615/critrevoncog.2014011922. Crit Rev Oncog. 2014. PMID: 25597351 Review.
Cited by
- Proteomic Analysis of Hepatocellular Carcinoma Tissues With Encapsulation Shows Up-regulation of Leucine Aminopeptidase 3 and Phosphoenolpyruvate Carboxykinase 2.
Kuhara K, Kitagawa T, Baron B, Tokuda K, Sakamoto K, Nagano H, Nakamura K, Kobayashi M, Nagayasu H, Kuramitsu Y. Kuhara K, et al. Cancer Genomics Proteomics. 2021 May-Jun;18(3):307-316. doi: 10.21873/cgp.20261. Cancer Genomics Proteomics. 2021. PMID: 33893083 Free PMC article. - Reduction of Raf kinase inhibitor protein expression by Bcr-Abl contributes to chronic myelogenous leukemia proliferation.
Takemura T, Nakamura S, Yokota D, Hirano I, Ono T, Shigeno K, Fujisawa S, Ohnishi K. Takemura T, et al. J Biol Chem. 2010 Feb 26;285(9):6585-94. doi: 10.1074/jbc.M109.075788. Epub 2009 Dec 22. J Biol Chem. 2010. PMID: 20028985 Free PMC article. - The RAF Kinase Inhibitor Protein (RKIP): Good as Tumour Suppressor, Bad for the Heart.
Abd Alla J, Quitterer U. Abd Alla J, et al. Cells. 2022 Feb 14;11(4):654. doi: 10.3390/cells11040654. Cells. 2022. PMID: 35203304 Free PMC article. Review. - Protein alterations in infiltrating ductal carcinomas of the breast as detected by nonequilibrium pH gradient electrophoresis and mass spectrometry.
Kabbage M, Chahed K, Hamrita B, Guillier CL, Trimeche M, Remadi S, Hoebeke J, Chouchane L. Kabbage M, et al. J Biomed Biotechnol. 2008;2008:564127. doi: 10.1155/2008/564127. J Biomed Biotechnol. 2008. PMID: 18401453 Free PMC article.
References
- Alessi D R, Cohen P, Ashworth A, Cowley S, Leevers S J, Marshall C J. Assay and expression of mitogen-activated protein kinase, MAP kinase kinase, and Raf. Methods Enzymol. 1995;255:279–290. - PubMed
- Denouel-Galy A, Douville E M, Warne P H, Papin C, Laugier D, Calothy G, Downward J, Eychene A. Murine Ksr interacts with MEK and inhibits Ras-induced transformation. Curr Biol. 1998;8:46–55. - PubMed
- Downward J. KSR: a novel player in the RAS pathway. Cell. 1995;83:831–834. - PubMed
- Elion E A. Routing MAP kinase cascades. Science. 1998;281:1625–1626. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous