Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2 - PubMed (original) (raw)
Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2
X Wu et al. Proc Natl Acad Sci U S A. 2000.
Abstract
PTEN is a tumor suppressor gene mutated in human cancers. Although many mutations target the phosphatase domain, others create a truncated protein lacking the C-terminal PDZ-binding motif or a protein that extends beyond the PDZ-binding motif. Using the yeast two-hybrid system, we isolated a membrane-associated guanylate kinase family protein with multiple PDZ domains [AIP-1 (atrophin interacting protein 1), renamed MAGI-2 (membrane associated guanylate kinase inverted-2)]. MAGI-2 contains eight potential protein-protein interaction domains and is localized to tight junctions in the membrane of epithelial cells. PTEN binds to MAGI-2 through an interaction between the PDZ-binding motif of PTEN and the second PDZ domain of MAGI-2. MAGI-2 enhances the ability of PTEN to suppress Akt activation. Furthermore, certain PTEN mutants have reduced stability, which is restored by adding the minimal PDZ-binding motif back to the truncated protein. We propose that MAGI-2 improves the efficiency of PTEN signaling through assembly of a multiprotein complex at the cell membrane.
Figures
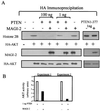
Figure 1
PTEN binds MAGI-2 through a PDZ domain-mediated interaction. (A) Structure of MAGI-2. The two clones isolated in the two-hybrid screen (clones 4.1 and 20.1) are indicated by arrows. PDZ0 indicates a probable PDZ domain that does not have the consensus GLGF sequence. The numbering of PDZ domains 1–5 is based on the previously published nomenclature for MAGI-1 (30). GuK, guanylate kinase domain. (B) 35S-labeled in vitro transcribed/translated MAGI-2 protein from clone 20.1 and clones of individual PDZ domains 2 or 4 was pulled down with GST alone or full-length PTEN-GST beads. Input represents 20% of the protein used. The bottom panel shows Coomassie staining of protein bound to beads. (C) 293T cells were transfected with wild-type (wt) or mutant FLAG-PTEN constructs and HA-MAGI-2. Lysates were immunoprecipitated (IP) with anti-FLAG antibody and immunoblotted with anti-HA antibody 12CA5 to detect MAGI-2 or anti-FLAG antibody to detect PTEN. (D) Transfection and immunoprecipitation (IP) were performed in 293T cells as indicated in C. (E) MAGI-2 was immunoprecipitated (IP) from the homogenate of fresh mouse brain (lane 4) by using antisera raised against the WW domains of S-SCAM (synaptic scaffolding molecule), the rat homologue of MAGI-2 (27). PTEN was immunoprecipitated (lane 5) by using polyclonal antisera (28) or an independently derived PTEN antibody (19) (not shown). Anti-Akt polyclonal antisera (New England Biolabs) (lane 3), pre-IP lysate (lane 6), and no lysate (lane 7) were used as controls. Immunoblots were analyzed by using rabbit polyclonal antisera against GST-MAGI-2.
Figure 2
Subcellular localization of PTEN and MAGI-2. MDCK cells were plated onto fibronectin-treated cover slips, then transfected with FLAG-PTEN or HA-MAGI-2. After 24 h the cells were washed, fixed in paraformaldehyde, permeabilized in 0.2% Triton X-100, and blocked in 3% BSA in PBS containing 1 mM CaCl2 and 1 mM MgCl2. Cells were incubated in primary antibody in blocking buffer for 1 h at 37°C. Cells were washed in blocking solution plus 0.2% Triton. Cells were incubated with secondary antibody in blocking buffer for 45 min, washed, and then visualized by using a Zeiss LSM310 laser scanning confocal microscope.
Figure 3
Effect of MAGI-2 on PTEN activity. (A) 293T cells were transfected with MAGI-2, HA-Akt, and different concentrations of wild-type or mutant PTEN plasmid as indicated. The Akt kinase activity was measured by immunoprecipitation using an anti-HA antibody, followed by a kinase assay using histone H2B as substrate. MAGI-2 and HA-Akt expression was verified by immunoblotting. (B) Quantitative results from two independent experiments are shown.
Figure 4
The PTEN PDZ-binding motif is required for complete Akt suppression. (A) Rat-1 fibroblasts stably expressing wild-type (WT) FLAG-PTEN, FLAG-PTEN 1–377, or Neo control were serum-starved overnight, then challenged with serum for 30 min. Phospho-Akt, total Akt, and PTEN levels were measured by immunoblot (16). (B) Pulse–chase experiments were performed in 293T cells by transfection with indicated plasmids. PTEN was immunoprecipitated by using anti-FLAG antibody and visualized by autoradiography. Signals quantitated by a PhosphorImager are shown relative to time 0 of the chase. (C) NIH 3T3 cells stably expressing indicated PTEN proteins were harvested after serum stimulation at indicated times and processed as described in A, except anti-PTEN antibody (Santa Cruz Biotechnology) was used to measure PTEN expression.
Figure 5
The PDZ binding motif of PTEN affects protein stability. (A) Schematic diagram of PTEN mutants. WT, wild type. (B) Coimmunoprecipitation experiments were performed in 293T cells transfected with the indicated plasmids as described in Fig. 1. IP, immunoprecipitation. (C) Rat-1 (Upper) and LNCaP (Lower) cells were infected with retrovirus expressing the indicated PTEN mutant, then selected in G418. Lysates were analyzed for expression of FLAG-PTEN at multiple time points. Results from the 25-day time point are shown.
Similar articles
- Binding of PTEN to specific PDZ domains contributes to PTEN protein stability and phosphorylation by microtubule-associated serine/threonine kinases.
Valiente M, Andrés-Pons A, Gomar B, Torres J, Gil A, Tapparel C, Antonarakis SE, Pulido R. Valiente M, et al. J Biol Chem. 2005 Aug 12;280(32):28936-43. doi: 10.1074/jbc.M504761200. Epub 2005 Jun 10. J Biol Chem. 2005. PMID: 15951562 - Interaction of the tumor suppressor PTEN/MMAC with a PDZ domain of MAGI3, a novel membrane-associated guanylate kinase.
Wu Y, Dowbenko D, Spencer S, Laura R, Lee J, Gu Q, Lasky LA. Wu Y, et al. J Biol Chem. 2000 Jul 14;275(28):21477-85. doi: 10.1074/jbc.M909741199. J Biol Chem. 2000. PMID: 10748157 - Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex.
Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. Vazquez F, et al. J Biol Chem. 2001 Dec 28;276(52):48627-30. doi: 10.1074/jbc.C100556200. Epub 2001 Nov 13. J Biol Chem. 2001. PMID: 11707428 - PTEN: from pathology to biology.
Sulis ML, Parsons R. Sulis ML, et al. Trends Cell Biol. 2003 Sep;13(9):478-83. doi: 10.1016/s0962-8924(03)00175-2. Trends Cell Biol. 2003. PMID: 12946627 Review. - Pten signaling in gliomas.
Knobbe CB, Merlo A, Reifenberger G. Knobbe CB, et al. Neuro Oncol. 2002 Jul;4(3):196-211. Neuro Oncol. 2002. PMID: 12084351 Free PMC article. Review.
Cited by
- Identification of regulatory SNPs associated with genetic modifications in lung adenocarcinoma.
Lu TP, Hsiao CK, Lai LC, Tsai MH, Hsu CP, Lee JM, Chuang EY. Lu TP, et al. BMC Res Notes. 2015 Mar 24;8:92. doi: 10.1186/s13104-015-1053-8. BMC Res Notes. 2015. PMID: 25889623 Free PMC article. - Evaluating the Role of MAST1 as an Intellectual Disability Disease Gene: Identification of a Novel De Novo Variant in a Patient with Developmental Disabilities.
Ben-Mahmoud A, Al-Shamsi AM, Ali BR, Al-Gazali L. Ben-Mahmoud A, et al. J Mol Neurosci. 2020 Mar;70(3):320-327. doi: 10.1007/s12031-019-01415-8. J Mol Neurosci. 2020. PMID: 31721002 - Evolution of metazoan cell junction proteins: the scaffold protein MAGI and the transmembrane receptor tetraspanin in the demosponge Suberites domuncula.
Adell T, Gamulin V, Perović-Ottstadt S, Wiens M, Korzhev M, Müller IM, Müller WE. Adell T, et al. J Mol Evol. 2004 Jul;59(1):41-50. doi: 10.1007/s00239-004-2602-2. J Mol Evol. 2004. PMID: 15383906 - MotifAnalyzer-PDZ: A computational program to investigate the evolution of PDZ-binding target specificity.
Valgardson J, Cosbey R, Houser P, Rupp M, Van Bronkhorst R, Lee M, Jagodzinski F, Amacher JF. Valgardson J, et al. Protein Sci. 2019 Dec;28(12):2127-2143. doi: 10.1002/pro.3741. Epub 2019 Nov 1. Protein Sci. 2019. PMID: 31599029 Free PMC article. - Epithelial cell polarity and tumorigenesis: new perspectives for cancer detection and treatment.
Coradini D, Casarsa C, Oriana S. Coradini D, et al. Acta Pharmacol Sin. 2011 May;32(5):552-64. doi: 10.1038/aps.2011.20. Epub 2011 Apr 18. Acta Pharmacol Sin. 2011. PMID: 21499288 Free PMC article. Review.
References
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. - PubMed
- Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, et al. Nat Genet. 1997;15:356–362. - PubMed
- Li D M, Sun H. Cancer Res. 1997;57:2124–2129. - PubMed
- Liaw D, Marsh D J, Li J, Dahia P L, Wang S I, Zheng Z, Bose S, Call K M, Tsou H C, Peacocke M, et al. Nat Genet. 1997;16:64–67. - PubMed
- Marsh D J, Dahia P L, Zheng Z, Liaw D, Parsons R, Gorlin R J, Eng C. Nat Genet. 1997;16:333–334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous