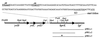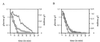Phosphorylation of HPr by the bifunctional HPr Kinase/P-ser-HPr phosphatase from Lactobacillus casei controls catabolite repression and inducer exclusion but not inducer expulsion - PubMed (original) (raw)
Phosphorylation of HPr by the bifunctional HPr Kinase/P-ser-HPr phosphatase from Lactobacillus casei controls catabolite repression and inducer exclusion but not inducer expulsion
V Dossonnet et al. J Bacteriol. 2000 May.
Abstract
We have cloned and sequenced the Lactobacillus casei hprK gene encoding the bifunctional enzyme HPr kinase/P-Ser-HPr phosphatase (HprK/P). Purified recombinant L. casei HprK/P catalyzes the ATP-dependent phosphorylation of HPr, a phosphocarrier protein of the phosphoenolpyruvate:carbohydrate phosphotransferase system at the regulatory Ser-46 as well as the dephosphorylation of seryl-phosphorylated HPr (P-Ser-HPr). The two opposing activities of HprK/P were regulated by fructose-1,6-bisphosphate, which stimulated HPr phosphorylation, and by inorganic phosphate, which stimulated the P-Ser-HPr phosphatase activity. A mutant producing truncated HprK/P was found to be devoid of both HPr kinase and P-Ser-HPr phosphatase activities. When hprK was inactivated, carbon catabolite repression of N-acetylglucosaminidase disappeared, and the lag phase observed during diauxic growth of the wild-type strain on media containing glucose plus either lactose or maltose was strongly diminished. In addition, inducer exclusion exerted by the presence of glucose on maltose transport in the wild-type strain was abolished in the hprK mutant. However, inducer expulsion of methyl beta-D-thiogalactoside triggered by rapidly metabolizable carbon sources was still operative in ptsH mutants altered at Ser-46 of HPr and the hprK mutant, suggesting that, in contrast to the model proposed for inducer expulsion in gram-positive bacteria, P-Ser-HPr might not be involved in this regulatory process.
Figures
FIG. 1
Schematic presentation of the 3,139-bp-long cloned and sequenced chromosomal L. casei DNA fragment containing the hprK gene. Indicated are the five ORFs (yvlB, yvlC, yvlD, hprK, and lgt) detected in this fragment and several restriction sites. The DNA sequence shown above this scheme represents the region preceding hprK and includes a putative promoter (−10 and −35), a presumed ribosome binding site (SD), and the ATG start codon. The L. casei DNA fragments present in the plasmids pHKLc1, pHKLc2, and pHKLc3 are aligned underneath the schematic presentation of the cloned L. casei DNA. The asterisk in pHKLc3 indicates the position of the hprK208(Am) mutation.
FIG. 2
[γ-32P]ATP-dependent phosphorylation of B. subtilis and L. casei HPr(His)6 with crude extracts prepared from either the L. casei wild-type strain (BL23) or the hprK208(Am) mutant (LcG102). Phosphorylation experiments were carried out with 1.5 μg of HPr as described in Materials and Methods. After electrophoresis, the 15% polyacrylamide gel containing 0.1% sodium dodecyl sulfate was treated with 16% boiling trichloroacetic acid, dried, and exposed to autoradiography. Lane 1, HPr from B. subtilis and crude extract from BL23; lane 2, HPr from B. subtilis and crude extract from LcG102; lane 3, HPr from L. casei and crude extract from BL23; lane 4, HPr from L. casei and crude extract from LcG102.
FIG. 3
The effect of FBP and Pi on ATP-dependent phosphorylation of B. subtilis HPr. HPr and P-Ser-HPr were separated on nondenaturing 12.5% polyacrylamide gels. (A) Effect of FBP on the L. casei HPr kinase activity. HPr phosphorylation was carried out with 20 ng of HprK/P and the indicated concentrations of FBP for 3 min at 37°C as described in Materials and Methods. HPr standard (2.5 μg) was loaded on lane 6. (B) Effect of Pi on L. casei P-Ser-HPr phosphatase activity. HprK/P-catalyzed dephosphorylation of P-Ser-HPr was performed with 50 ng of HprK/P and the indicated Pi concentrations by incubating the assay mixture for 5 min at 37°C as described in Materials and Methods. P-Ser-HPr standard (2.5 μg) was loaded on lane 1. (C) Effect of FBP on ATP-dependent HPr phosphorylation in the presence of Pi. ATP-dependent HPr phosphorylation was carried out for 5 min at 37°C with 20 ng of HprK/P and the indicated amounts of Pi in the presence (+) or absence (−) of 20 mM FBP. Lanes 10 and 11 contain 2.5 μg of HPr and P-Ser-HPr standards, respectively. After electrophoresis, gels were stained with Coomassie blue.
FIG. 4
P-Ser-HPr phosphatase activity in crude extracts of L. casei wild-type or hprK208(Am) mutant. P-Ser-HPr phosphatase assays were carried out with 10 μl of crude extracts in the absence (−) or presence (+) of 2.5 μg of P-Ser-HPr as described in Materials and Methods. P-Ser-HPr and HPr standards (2.5 μg each) were loaded on lanes 5 and 6, respectively. Samples were separated on a nondenaturing 12.5% polyacrylamide gel which was stained with Coomassie blue.
FIG. 5
[14C]Maltose uptake in the presence (diamonds) and absence (squares) of glucose by L. casei wild-type (A) and hprK208(Am) mutant (B) cells. Transport studies were carried out by using the rapid filtration method. Glucose at a final concentration of 1 mM was added at the time indicated by the arrow.
FIG. 6
Consumption of maltose (0.025%) and glucose (0.15%) by resting L. casei wild-type (A) or hprK208(Am) mutant (B) cells. The cell suspension containing 18 mg of cells (dry weight) in 5 ml of 50 mM sodium phosphate buffer, pH 7, was incubated at 37°C. Samples of 300 μl were withdrawn at the indicated time intervals and were centrifuged. The maltose concentration in experiments carried out in the presence (diamonds) or absence (circles) of glucose and the glucose concentration (squares) were determined in the supernatant.
FIG. 7
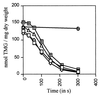
Expulsion of preaccumulated [14C]TMG-6-P in different L. casei strains. Cells preloaded with [14C]TMG-6-P were washed and resuspended in 1 ml of transport buffer. At time 0, glucose was added at a final concentration of 5 mM, and 100-μl aliquots were withdrawn at the indicated time intervals. The radioactivity remaining inside the cells was measured with the wild-type strain BL23 (squares) and the ptsH1 (triangles), the ptsH2 (cross within squares), and the hprK208(Am) (diamonds) mutant strains. Two control samples were taken before glucose was added. One was immediately filtered and provided time point 0, the other was incubated at 37°C and was filtered at the end of the experiment (crosses within circles). This end point is shown only for the experiment with the ptsH1 mutant. A similar leakage (less than 5%) of [14C]TMG-6-P in the absence of glucose was observed with all other strains.
Similar articles
- Enzyme I and HPr from Lactobacillus casei: their role in sugar transport, carbon catabolite repression and inducer exclusion.
Viana R, Monedero V, Dossonnet V, Vadeboncoeur C, Pérez-Martínez G, Deutscher J. Viana R, et al. Mol Microbiol. 2000 May;36(3):570-84. doi: 10.1046/j.1365-2958.2000.01862.x. Mol Microbiol. 2000. PMID: 10844647 - Pyrophosphate-producing protein dephosphorylation by HPr kinase/phosphorylase: a relic of early life?
Mijakovic I, Poncet S, Galinier A, Monedero V, Fieulaine S, Janin J, Nessler S, Marquez JA, Scheffzek K, Hasenbein S, Hengstenberg W, Deutscher J. Mijakovic I, et al. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13442-7. doi: 10.1073/pnas.212410399. Epub 2002 Oct 1. Proc Natl Acad Sci U S A. 2002. PMID: 12359880 Free PMC article. - HPr kinase/phosphorylase, a Walker motif A-containing bifunctional sensor enzyme controlling catabolite repression in Gram-positive bacteria.
Poncet S, Mijakovic I, Nessler S, Gueguen-Chaignon V, Chaptal V, Galinier A, Boël G, Mazé A, Deutscher J. Poncet S, et al. Biochim Biophys Acta. 2004 Mar 11;1697(1-2):123-35. doi: 10.1016/j.bbapap.2003.11.018. Biochim Biophys Acta. 2004. PMID: 15023355 Review. - Mutations lowering the phosphatase activity of HPr kinase/phosphatase switch off carbon metabolism.
Monedero V, Poncet S, Mijakovic I, Fieulaine S, Dossonnet V, Martin-Verstraete I, Nessler S, Deutscher J. Monedero V, et al. EMBO J. 2001 Aug 1;20(15):3928-37. doi: 10.1093/emboj/20.15.3928. EMBO J. 2001. PMID: 11483496 Free PMC article. - Transcription regulators potentially controlled by HPr kinase/phosphorylase in Gram-negative bacteria.
Boël G, Mijakovic I, Mazé A, Poncet S, Taha MK, Larribe M, Darbon E, Khemiri A, Galinier A, Deutscher J. Boël G, et al. J Mol Microbiol Biotechnol. 2003;5(4):206-15. doi: 10.1159/000071072. J Mol Microbiol Biotechnol. 2003. PMID: 12867744 Review.
Cited by
- X-ray structure of HPr kinase: a bacterial protein kinase with a P-loop nucleotide-binding domain.
Fieulaine S, Morera S, Poncet S, Monedero V, Gueguen-Chaignon V, Galinier A, Janin J, Deutscher J, Nessler S. Fieulaine S, et al. EMBO J. 2001 Aug 1;20(15):3917-27. doi: 10.1093/emboj/20.15.3917. EMBO J. 2001. PMID: 11483495 Free PMC article. - Development of genetic tools for Lactobacillus sakei: disruption of the beta-galactosidase gene and use of lacZ as a reporter gene To study regulation of the putative copper ATPase, AtkB.
Stentz R, Loizel C, Malleret C, Zagorec M. Stentz R, et al. Appl Environ Microbiol. 2000 Oct;66(10):4272-8. doi: 10.1128/AEM.66.10.4272-4278.2000. Appl Environ Microbiol. 2000. PMID: 11010870 Free PMC article. - YvoA and CcpA Repress the Expression of chiB in Bacillus thuringiensis.
Jiang K, Li LN, Pan JH, Wang TT, Chen YH, Cai J. Jiang K, et al. Appl Environ Microbiol. 2015 Oct;81(19):6548-57. doi: 10.1128/AEM.01549-15. Epub 2015 Jul 10. Appl Environ Microbiol. 2015. PMID: 26162881 Free PMC article. - High-resolution structure of the histidine-containing phosphocarrier protein (HPr) from Staphylococcus aureus and characterization of its interaction with the bifunctional HPr kinase/phosphorylase.
Maurer T, Meier S, Kachel N, Munte CE, Hasenbein S, Koch B, Hengstenberg W, Kalbitzer HR. Maurer T, et al. J Bacteriol. 2004 Sep;186(17):5906-18. doi: 10.1128/JB.186.17.5906-5918.2004. J Bacteriol. 2004. PMID: 15317796 Free PMC article. - Identification of HPr kinase/phosphorylase inhibitors: novel antimicrobials against resistant Enterococcus faecalis.
Kumar S, Bhadane R, Shandilya S, Salo-Ahen OMH, Kapila S. Kumar S, et al. J Comput Aided Mol Des. 2022 Jul;36(7):507-520. doi: 10.1007/s10822-022-00461-6. Epub 2022 Jul 9. J Comput Aided Mol Des. 2022. PMID: 35809194 Free PMC article.
References
- Deutscher J, Küster E, Bergstedt U, Charrier V, Hillen W. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in Gram-positive bacteria. Mol Microbiol. 1995;15:1049–1053. - PubMed
- Deutscher J, Pevec B, Beyreuther K, Kiltz H-H, Hengstenberg W. Streptococcal phosphoenolpyruvate-sugar phosphotransferase system: amino acid sequence and site of ATP-dependent phosphorylation of HPr. Biochemistry. 1986;25:6543–6551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources