Danish type gelsolin related amyloidosis: 654G-T mutation is associated with a disease pathogenetically and clinically similar to that caused by the 654G-A mutation (familial amyloidosis of the Finnish type) - PubMed (original) (raw)
Danish type gelsolin related amyloidosis: 654G-T mutation is associated with a disease pathogenetically and clinically similar to that caused by the 654G-A mutation (familial amyloidosis of the Finnish type)
C P Maury et al. J Clin Pathol. 2000 Feb.
Abstract
Background: Familial amyloidosis of the Finnish type (FAF, Finnish hereditary amyloidosis) is caused by a 654G-A mutation in the gelsolin gene on chromosome 9 resulting in the expression of mutant Asn-187 gelsolin which is abnormally proteolytically processed generating amyloidogenic fragments that polymerize into amyloid fibrils. We have recently shown that in a Danish and a Czech family with a clinical syndrome similar to FAF, including corneal lattice dystrophy, cranial neuropathy and skin changes, the disease is caused by another mutation at the same position, namely 654G-T predicting a Try-for-Asp substitution at 187 in secreted gelsolin.
Aim: To undertake a closer examination of the Danish subtype of FAF and report immunohistochemical and biochemical findings.
Results: Immunostaining of plasma gelsolin isolated from heterozygous FAF of the Danish subtype revealed a pattern similar to that found in FAF-Asn 187. The > 60 kDa gelsolin species contain an epitope characteristic of the amyloid forming region as revealed by an amyloid specific antibody, whereas the approximately 50 kDa fragments are devoid of it. Compared with the wild-type gelsolin peptide (Asp-187), the corresponding mutant peptide (Tyr-187) showed dramatically increased fibrillogenicity as revealed by quantitative thioflavine-T based fluorimetry; ultrastructurally, amyloid-like fibrils were formed by the mutant peptide. Immunohistochemistry showed that antibodies directed against residues 231-242 of secreted gelsolin, representing the carboxy terminus of the sequence forming the amyloid protein (residues 173-243) laid down in the tissues in a fibrillar form in FAF, specifically labelled the amyloid deposited in rectum and skin in the Danish (654G-T) subtype.
Conclusions: The 654G-T mutation in the gelsolin gene gives rise to an amyloid disease clinically and pathogenetically similar to that caused by the 654G-A mutation.
Figures
Figure 1 Schematic presentation of the amyloid forming region (residues 173–243) and mutation site in secreted gelsolin (residues 1–755) in Danish-type FAF, as well as the regions of the gelsolin molecule to which the amyloid specific polyclonal antibody (PoAb 231–242) and the monoclonal antibody MoAb-2C4 are directed.
Figure 2 Reverse phase high pressure liquid chromatography on a Vydac C18 column of synthetic mutant 182–192 gelsolin peptide (Tyr-187).
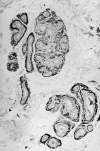
Figure 3 Dermal eccrine glands in Danish subtype (Tyr-187) familial amyloidosis showing prominent amyloid deposits in thickened basement membranes. Congo red staining, polarised light, magnification x140.
Figure 4 Immunogold-silver staining with amyloid specific antibody PoAb 231–242 of a skin biopsy in Danish subtype (Tyr-187) amyloidosis. Amyloid located in basement membranes is specifically stained. Magnification x134.
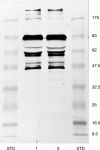
Figure 5 Comparison of heterozygous Danish subtype FAF (Tyr-187) and heterozygous Asn-187 FAF by immunoblotting by the monoconal MoAb-2C4 antibody. Lane 1, FAF Asn-187; lane 2, Danish FAF (Tyr-187); STD, prestained molecular weight markers (kDa).
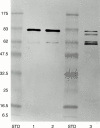
Figure 6 Immunostaining of the gelsolin fraction isolated from FAF plasma by affinity chromatography on an MoAb-2C4 Sepharose column by polyclonal antiamyloid antibodies directed against the C terminus of the amyloid region (231–242). Lane 1, heterozygous FAF Tyr-187 (Danish); lane 2, heterozygous FAF Asn-187; lane 3, homozygous FAF Asn-187; STD, prestained molecular weight markers (kDa). Note that the ∼50 kDa bands (fig 5 ) are not stained by the amyloid specific antibody as they are devoid of the amyloid forming sequence.
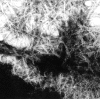
Figure 7 Electron micrograph of negatively stained protein assemblies formed by the mutant (Tyr-187) peptide, corresponding to residues 182–192 in gelsolin. Magnification x59 595.
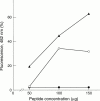
Figure 8 Comparison of amyloid fibril formation at different peptide concentrations by mutant (Tyr-187) gelsolin 182–192 peptide corresponding to Danish type amyloidosis (filled triangles), by mutant (Asn-187) gelsolin peptide 182–192 corresponding to Finnish amyloidosis (empty circles), and wild-type (Asp-187) gelsolin 182–192 peptide (filled circles), as measured by quantitative thioflavine-T based fluorimetry.
Similar articles
- Fibrillogenesis in gelsolin-related familial amyloidosis.
Maury CP, Nurmiaho-Lassila EL, Boysen G, Liljeström M. Maury CP, et al. Amyloid. 2003 Aug;10 Suppl 1:21-5. Amyloid. 2003. PMID: 14640038 - Danish type gelsolin-related amyloidosis in a Brazilian family: case reports.
Solari HP, Ventura MP, Antecka E, Belfort Junior R, Burnier MN Jr. Solari HP, et al. Arq Bras Oftalmol. 2011 Jul-Aug;74(4):286-8. doi: 10.1590/s0004-27492011000400012. Arq Bras Oftalmol. 2011. PMID: 22068858 - Gelsolin amyloidosis: genetics, biochemistry, pathology and possible strategies for therapeutic intervention.
Solomon JP, Page LJ, Balch WE, Kelly JW. Solomon JP, et al. Crit Rev Biochem Mol Biol. 2012 May-Jun;47(3):282-96. doi: 10.3109/10409238.2012.661401. Epub 2012 Feb 24. Crit Rev Biochem Mol Biol. 2012. PMID: 22360545 Free PMC article. Review. - Ca2+ binding protects against gelsolin amyloidosis.
Page LJ, Huff ME, Kelly JW, Balch WE. Page LJ, et al. Biochem Biophys Res Commun. 2004 Oct 1;322(4):1105-10. doi: 10.1016/j.bbrc.2004.07.125. Biochem Biophys Res Commun. 2004. PMID: 15336957 Review.
Cited by
- Clinical features and haplotype analysis of newly identified Japanese patients with gelsolin-related familial amyloidosis of Finnish type.
Taira M, Ishiura H, Mitsui J, Takahashi Y, Hayashi T, Shimizu J, Matsukawa T, Saito N, Okada K, Tsuji S, Sawamura H, Amano S, Goto J, Tsuji S. Taira M, et al. Neurogenetics. 2012 Aug;13(3):237-43. doi: 10.1007/s10048-012-0330-0. Epub 2012 May 24. Neurogenetics. 2012. PMID: 22622774 - Clinical and Histopathological Features of Gelsolin Amyloidosis Associated with a Novel GSN Variant p.Glu580Lys.
Potrč M, Volk M, de Rosa M, Pižem J, Teran N, Jaklič H, Maver A, Drnovšek-Olup B, Bollati M, Vogelnik K, Hočevar A, Gornik A, Pfeifer V, Peterlin B, Hawlina M, Fakin A. Potrč M, et al. Int J Mol Sci. 2021 Jan 22;22(3):1084. doi: 10.3390/ijms22031084. Int J Mol Sci. 2021. PMID: 33499149 Free PMC article. - [Hereditary and non-hereditary cutaneous amyloidoses].
Schreml S, Schroeder J, Eder F, Szeimies RM, Landthaler M, Babilas P. Schreml S, et al. Pathologe. 2009 May;30(3):197-204. doi: 10.1007/s00292-009-1130-7. Pathologe. 2009. PMID: 19319536 Review. German. - Furin at the cutting edge: from protein traffic to embryogenesis and disease.
Thomas G. Thomas G. Nat Rev Mol Cell Biol. 2002 Oct;3(10):753-66. doi: 10.1038/nrm934. Nat Rev Mol Cell Biol. 2002. PMID: 12360192 Free PMC article. Review. - Clinical, histopathological, and in silico pathogenicity analyses in a pedigree with familial amyloidosis of the Finnish type (Meretoja syndrome) caused by a novel gelsolin mutation.
Cabral-Macias J, Garcia-Montaño LA, Pérezpeña-Díazconti M, Aguilar MC, Garcia G, Vencedor-Meraz CI, Graue-Hernandez EO, Chacón-Camacho OF, Zenteno JC. Cabral-Macias J, et al. Mol Vis. 2020 May 2;26:345-354. eCollection 2020. Mol Vis. 2020. PMID: 32368002 Free PMC article.
References
- Ann Clin Res. 1969 Dec;1(4):314-24 - PubMed
- J Exp Med. 1990 Dec 1;172(6):1865-7 - PubMed
- Anal Biochem. 1989 Mar;177(2):244-9 - PubMed
- FEBS Lett. 1990 Dec 10;276(1-2):75-7 - PubMed
- Biochim Biophys Acta. 1990 Nov 14;1096(1):84-6 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials