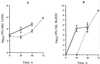Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae - PubMed (original) (raw)
Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae
H Yesilkaya et al. Infect Immun. 2000 May.
Abstract
Streptococcus pneumoniae was shown to contain two types of superoxide dismutase, MnSOD and FeSOD. Levels of MnSOD increased during growth in an aerobic environment. The sodA gene, encoding MnSOD, of virulent S. pneumoniae type 2 strain D39 was inactivated to give mutant D39HY1. Aerobically, D39HY1 had a lower growth rate than the wild type and exhibited susceptibility to the redox-active compound paraquat, but anaerobic growth of D39HY1 was identical to that of the wild type. Virulence studies showed that the median survival time of mice infected intranasally with D39HY1 was significantly longer than that of mice infected with the wild-type pneumococcus. In contrast to the wild type, D39HY1 did not multiply in lungs during the first 24 h but thereafter grew at the same rate as the wild type. Appearance in the bloodstream was also delayed, but growth in the blood was unimpaired by the sodA mutation. The pattern of inflammation in lungs infected with D39HY1 differed from that in wild-type-infected mice. After infection with D39HY1, neutrophils were densely packed around bronchioles, in contrast to the wild-type infection, where neutrophils were more diffusely localized.
Figures
FIG. 1
Type inhibition assay and insertional inactivation of SOD activity. Nondenaturing PAGE gels for determination of SOD activity were treated (B) or not treated (A) with 5 mM H2O2. (A and B) Lanes 1 contain E. coli extract and lanes 2 contain S. pneumoniae extract. (C) Extract of the wild type (lane 2) or mutant (D39HY1) (lane 1) was separated on nondenaturing PAGE gels. The lane containing the wild type had two bands of activity, a minor and a major band, whereas D39HY1 had only the minor activity band. Arrows indicate the minor and major SODs.
FIG. 2
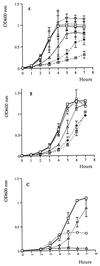
In vitro growth of wild type and sod mutant strain (D39HY1). Aerobic growth (A) and anaerobic growth (B) in BHI broth with and without paraquat and growth in defined medium (C) are shown. (A and B) Strains and concentrations of paraquat are represented with symbols as follows: wild type with no paraquat ( ), wild type with 0.001 M paraquat (
), wild type with 0.001 M paraquat ( ), wild type with 0.0001 M paraquat (
), wild type with 0.0001 M paraquat ( ), D39HY1 with no paraquat (
), D39HY1 with no paraquat ( ), D39HY1 with 0.001 M paraquat (
), D39HY1 with 0.001 M paraquat ( ), and D39HY1 with 0.0001 M paraquat (
), and D39HY1 with 0.0001 M paraquat ( ). (C) Strains and growth conditions are represented with symbols as follows: wild type, anaerobic conditions (
). (C) Strains and growth conditions are represented with symbols as follows: wild type, anaerobic conditions ( ); wild type, aerobic conditions (
); wild type, aerobic conditions ( ); D39HY1, anaerobic conditions (
); D39HY1, anaerobic conditions ( ); and D39HY1, aerobic conditions (
); and D39HY1, aerobic conditions ( ). Each point is the mean of three experiments.
). Each point is the mean of three experiments.
FIG. 3
Survival of mice infected intranasally with D39HY1 or the wild-type (WT) pneumococcus. Symbols show the time that individual mice became moribund. The horizontal bars mark the median time to the moribund state.
FIG. 4
Time course of bacterial growth in the lungs (A) and blood (B) of mice infected intranasally with D39HY1 or the wild-type pneumococcus. Symbols indicate mice infected with the wild type ( ) or D39HY1 (
) or D39HY1 ( ). Each point is the mean log data from five MF1 female mice except 72 h, which is from three mice. Error bars show the standard errors of the means.
). Each point is the mean log data from five MF1 female mice except 72 h, which is from three mice. Error bars show the standard errors of the means.
FIG. 5
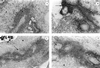
Lung histology. Light microscopy of cryostat cut frozen sections (20 μm) of lung tissue from mice infected with 106 wild-type bacteria and sacrificed at 24 h (panel A; magnification, ×400; large arrow, bronchiole wall; pair of small arrows, cellular infiltration; open arrowhead, perivascular space) and 48 h (panel B; magnification, ×400; large arrow, heavy cellular infiltration; pair of large arrows, fibrosis; pair of small arrows, edema; open arrowhead, bronchiole wall) and from mice infected with 106 D39HY1 bacteria and sacrificed at 24 h (panel C; magnification, ×300; large arrow, bronchiole wall; pair of small arrows, heavy cellular infiltration; open arrowhead, perivascular space) and 48 h (panel D; magnification, ×400; large arrow, bronchiole wall; pair of small arrows, heavy cellular infiltration; open arrowhead, perivascular space). Sections were stained with hematoxylin and eosin.
Similar articles
- The pneumococcal response to oxidative stress includes a role for Rgg.
Bortoni ME, Terra VS, Hinds J, Andrew PW, Yesilkaya H. Bortoni ME, et al. Microbiology (Reading). 2009 Dec;155(Pt 12):4123-4134. doi: 10.1099/mic.0.028282-0. Epub 2009 Sep 17. Microbiology (Reading). 2009. PMID: 19762446 Free PMC article. - Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress.
Tseng HJ, McEwan AG, Paton JC, Jennings MP. Tseng HJ, et al. Infect Immun. 2002 Mar;70(3):1635-9. doi: 10.1128/IAI.70.3.1635-1639.2002. Infect Immun. 2002. PMID: 11854257 Free PMC article. - Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae.
Poyart C, Pellegrini E, Gaillot O, Boumaila C, Baptista M, Trieu-Cuot P. Poyart C, et al. Infect Immun. 2001 Aug;69(8):5098-106. doi: 10.1128/IAI.69.8.5098-5106.2001. Infect Immun. 2001. PMID: 11447191 Free PMC article. - Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae.
Giammarinaro P, Paton JC. Giammarinaro P, et al. Infect Immun. 2002 Oct;70(10):5454-61. doi: 10.1128/IAI.70.10.5454-5461.2002. Infect Immun. 2002. PMID: 12228270 Free PMC article.
Cited by
- Copper intoxication inhibits aerobic nucleotide synthesis in Streptococcus pneumoniae.
Johnson MD, Kehl-Fie TE, Rosch JW. Johnson MD, et al. Metallomics. 2015 May;7(5):786-94. doi: 10.1039/c5mt00011d. Epub 2015 Mar 2. Metallomics. 2015. PMID: 25730343 Free PMC article. - The oxidative stress response of Streptococcus pneumoniae: its contribution to both extracellular and intracellular survival.
Hernandez-Morfa M, Olivero NB, Zappia VE, Piñas GE, Reinoso-Vizcaino NM, Cian MB, Nuñez-Fernandez M, Cortes PR, Echenique J. Hernandez-Morfa M, et al. Front Microbiol. 2023 Sep 13;14:1269843. doi: 10.3389/fmicb.2023.1269843. eCollection 2023. Front Microbiol. 2023. PMID: 37789846 Free PMC article. Review. - Identification of Streptococcus pneumoniae genes associated with hypothiocyanous acid tolerance through genome-wide screening.
Shearer HL, Pace PE, Smith LM, Fineran PC, Matthews AJ, Camilli A, Dickerhof N, Hampton MB. Shearer HL, et al. J Bacteriol. 2023 Oct 26;205(10):e0020823. doi: 10.1128/jb.00208-23. Epub 2023 Oct 4. J Bacteriol. 2023. PMID: 37791755 Free PMC article. - Dual Role of Hydrogen Peroxide as an Oxidant in Pneumococcal Pneumonia.
Mraheil MA, Toque HA, La Pietra L, Hamacher J, Phanthok T, Verin A, Gonzales J, Su Y, Fulton D, Eaton DC, Chakraborty T, Lucas R. Mraheil MA, et al. Antioxid Redox Signal. 2021 Apr 20;34(12):962-978. doi: 10.1089/ars.2019.7964. Epub 2020 Aug 14. Antioxid Redox Signal. 2021. PMID: 32283950 Free PMC article. Review. - The therapeutic potential of thiocyanate and hypothiocyanous acid against pulmonary infections.
Ashtiwi NM, Kim SO, Chandler JD, Rada B. Ashtiwi NM, et al. Free Radic Biol Med. 2024 Jul;219:104-111. doi: 10.1016/j.freeradbiomed.2024.04.217. Epub 2024 Apr 11. Free Radic Biol Med. 2024. PMID: 38608822 Review.
References
- Andrew P W, Mitchell T J, Myint S H. Prevention of respiratory infections. Curr Opin Infect Dis. 1993;6:146–149.
- Andrew P W, Boulnois G J, Mitchell T J, Lee C J, Paton J C, Poolman J T, Peeters C C A M. Pneumococcal vaccines. Zentbl Bakteriol. 1994;24:453–466.
- Beauchamp C O, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. - PubMed
- Beyer W F, Fridovich I. In vivo competition between iron and manganese for occupancy of the active site region of the manganese-superoxide dismutase of Escherichia coli. J Biol Chem. 1991;266:303–308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources