Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host-pathogen interactions - PubMed (original) (raw)
Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host-pathogen interactions
J M Solomon et al. Infect Immun. 2000 May.
Abstract
Conditions were established in which Legionella pneumophila, an intracellular bacterial pathogen, could replicate within the unicellular organism Dictyostelium discoideum. By several criteria, L. pneumophila grew by the same mechanism within D. discoideum as it does in amoebae and macrophages. Bacteria grew within membrane-bound vesicles associated with rough endoplasmic reticulum, and L. pneumophila dot/icm mutants, blocked for growth in macrophages and amoebae, also did not grow in D. discoideum. Internalized L. pneumophila avoided degradation by D. discoideum and showed evidence of reduced fusion with endocytic compartments. The ability of L. pneumophila to grow within D. discoideum depended on the growth state of the cells. D. discoideum grown as adherent monolayers was susceptible to L. pneumophila infection and to contact-dependent cytotoxicity during high-multiplicity infections, whereas D. discoideum grown in suspension was relatively resistant to cytotoxicity and did not support intracellular growth. Some known D. discoideum mutants were examined for their effect on growth of L. pneumophila. The coronin mutant and the myoA/B double myosin I mutant were more permissive than wild-type strains for intracellular growth. Growth of L. pneumophila in a G(beta) mutant was slightly reduced compared to the parent strain. This work demonstrates the usefulness of the L. pneumophila-D. discoideum system for genetic analysis of host-pathogen interactions.
Figures
FIG. 1
Growth of L. pneumophila in the presence of D. discoideum in liquid culture. D. discoideum was plated into tissue culture wells in MB medium. Cells were infected with wild-type L. pneumophila Philadelphia-1 at an MOI of 1:1. L. pneumophila and D. discoideum were counted by measuring CFU and PFU, respectively. The experiment was performed twice, each point in the experiment was done in triplicate, and the error bars indicate n − 1 weighted sample standard deviation.
FIG. 2
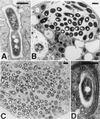
Transmission electron microscopy of D. discoideum infected with L. pneumophila. D. discoideum was infected with L. pneumophila Philadelphia-1 as in Fig. 1. On day 3, cells were harvested and prepared for electron microscopy. (A) Phagosome containing a bacterium apparently in the process of dividing; (B) vacuole containing a few bacteria; (C) a cell nearly taken over by a bacterium-filled phagosome; (D) multiple layers of RER associated with an L. pneumophila phagosome. Association of ribosomes with phagosomes can also be seen in panels A and B. In all panels, the bar equals 0.5 μm.
FIG. 3
Growth of wild-type and isogenic dot mutant L. pneumophila in D. discoideum. D. discoideum was plated in tissue culture wells in MB medium and infected with L. pneumophila at an MOI of 1:1. The number of viable bacteria was determined by counting CFU. The experiment was performed three times, each point in the experiment was done in triplicate, and the error bars indicate the n − 1 weighted sample standard deviation.
FIG. 4
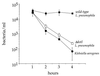
Viability of bacteria internalized by D. discoideum 1 to 4 h after infection. D. discoideum was plated in tissue culture wells in MB medium and infected with bacteria at an MOI of 5:1. After 30 min, gentamicin was added to kill extracellular bacteria. The number of viable intracellular bacteria remaining was counted by measuring CFU extracted from washed cells. The experiment was performed two times, each point was done in duplicate, and the error bars indicate the n − 1 weighted sample standard deviation.
FIG. 5
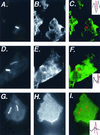
Association of lysosomal membrane proteins with _L. pneumophila_-containing phagosomes in D. discoideum. D. discoideum was plated on coverslips in tissue culture wells in MB medium and infected with L. pneumophila at an MOI of 10:1. After 30 min of infection, cells were fixed for immunofluoresence analysis. Cells in panels A to C and D to F were infected by dotI mutant L. pneumophila; cells in panels G to I were infected with dot+ L. pneumophila. Panels A, D, and G show staining of the bacteria; panels B, E, and H show staining of the anti-lysosomal membrane protein antibody. In panels C, F, and I, the two images are superimposed, with bacterial staining shown in red and lysosomal membrane protein staining shown in green. The inset indicates the bacterial fluorescence (red) or the lysosomal membrane protein fluorescence (black) along the line drawn in each panel. Images were processed with IP Lab Spectrum version 3.2.
FIG. 6
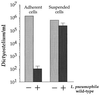
Susceptibility of adherent and suspended D. discoideum to a high-MOI infection of L. pneumophila. D. discoideum was incubated in MB medium either as adherent monolayers in tissue culture wells or as suspended cells shaken in tubes. Cells were infected (or not) with L. pneumophila Philadelphia-1 at an MOI of 375:1 for approximately 24 h. Viable D. discoideum cells were counted by measuring PFU. The experiment was performed twice, each condition was done in duplicate, and the error bars indicate the n − 1 weighted sample standard deviation.
FIG. 7
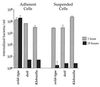
Survival of internalized bacteria in adherent and suspended D. discoideum. D. discoideum was incubated in MB medium either as adherent monolayers in tissue culture wells or as suspended cells shaken in tubes. Cells were infected with bacteria for 30 min, at which point gentamicin was added to kill extracellular bacteria. Viable, intracellular bacteria remaining were counted by measuring CFU extracted from washed cells. The experiment was performed twice, each condition was done in duplicate, and the error bars indicate the n − 1 weighted sample standard deviation.
FIG. 8
Growth of D. discoideum on lawns of L. pneumophila 105 D. discoideum were spotted onto bacterial lawns grown on CYE plates made with reduced concentrations of cysteine and iron. (A) Lawn of L. pneumophila strain Lp01 (dot+); (B) lawn of L. pneumophila strain HL056 (Δ_dotI_).
FIG. 9
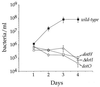
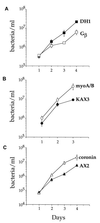
Growth of L. pneumophila in D. discoideum mutants. Various D. discoideum mutants and their parent strains were plated in tissue culture wells in MB medium and infected with L. pneumophila Philadelphia-1 at an MOI of 1:1. The number of viable bacteria was determined by counting CFU. The experiments were performed two to four times depending on the strain, each point in the experiment was done in triplicate, and the error bars indicate the n − 1 weighted sample standard deviation.
Similar articles
- Growth of Legionella pneumophila in Dictyostelium discoideum: a novel system for genetic analysis of host-pathogen interactions.
Solomon JM, Isberg RR. Solomon JM, et al. Trends Microbiol. 2000 Oct;8(10):478-80. doi: 10.1016/s0966-842x(00)01852-7. Trends Microbiol. 2000. PMID: 11044684 Review. - Dictyostelium discoideum strains lacking the RtoA protein are defective for maturation of the Legionella pneumophila replication vacuole.
Li Z, Solomon JM, Isberg RR. Li Z, et al. Cell Microbiol. 2005 Mar;7(3):431-42. doi: 10.1111/j.1462-5822.2004.00472.x. Cell Microbiol. 2005. PMID: 15679845 - The Legionella pneumophila response regulator LqsR promotes host cell interactions as an element of the virulence regulatory network controlled by RpoS and LetA.
Tiaden A, Spirig T, Weber SS, Brüggemann H, Bosshard R, Buchrieser C, Hilbi H. Tiaden A, et al. Cell Microbiol. 2007 Dec;9(12):2903-20. doi: 10.1111/j.1462-5822.2007.01005.x. Epub 2007 Jul 5. Cell Microbiol. 2007. PMID: 17614967 - Macroautophagy is dispensable for intracellular replication of Legionella pneumophila in Dictyostelium discoideum.
Otto GP, Wu MY, Clarke M, Lu H, Anderson OR, Hilbi H, Shuman HA, Kessin RH. Otto GP, et al. Mol Microbiol. 2004 Jan;51(1):63-72. doi: 10.1046/j.1365-2958.2003.03826.x. Mol Microbiol. 2004. PMID: 14651611 - Legionella pneumophila adaptation to intracellular life and the host response: clues from genomics and transcriptomics.
Jules M, Buchrieser C. Jules M, et al. FEBS Lett. 2007 Jun 19;581(15):2829-38. doi: 10.1016/j.febslet.2007.05.026. Epub 2007 May 21. FEBS Lett. 2007. PMID: 17531986 Review.
Cited by
- SdhA blocks disruption of the Legionella-containing vacuole by hijacking the OCRL phosphatase.
Choi WY, Kim S, Aurass P, Huo W, Creasey EA, Edwards M, Lowe M, Isberg RR. Choi WY, et al. Cell Rep. 2021 Nov 2;37(5):109894. doi: 10.1016/j.celrep.2021.109894. Cell Rep. 2021. PMID: 34731604 Free PMC article. - Multiple Legionella pneumophila Type II secretion substrates, including a novel protein, contribute to differential infection of the amoebae Acanthamoeba castellanii, Hartmannella vermiformis, and Naegleria lovaniensis.
Tyson JY, Pearce MM, Vargas P, Bagchi S, Mulhern BJ, Cianciotto NP. Tyson JY, et al. Infect Immun. 2013 May;81(5):1399-410. doi: 10.1128/IAI.00045-13. Epub 2013 Feb 19. Infect Immun. 2013. PMID: 23429532 Free PMC article. - Bacterial discrimination by dictyostelid amoebae reveals the complexity of ancient interspecies interactions.
Nasser W, Santhanam B, Miranda ER, Parikh A, Juneja K, Rot G, Dinh C, Chen R, Zupan B, Shaulsky G, Kuspa A. Nasser W, et al. Curr Biol. 2013 May 20;23(10):862-72. doi: 10.1016/j.cub.2013.04.034. Epub 2013 May 9. Curr Biol. 2013. PMID: 23664307 Free PMC article. - Aggravating genetic interactions allow a solution to redundancy in a bacterial pathogen.
O'Connor TJ, Boyd D, Dorer MS, Isberg RR. O'Connor TJ, et al. Science. 2012 Dec 14;338(6113):1440-4. doi: 10.1126/science.1229556. Science. 2012. PMID: 23239729 Free PMC article. - Immune-like phagocyte activity in the social amoeba.
Chen G, Zhuchenko O, Kuspa A. Chen G, et al. Science. 2007 Aug 3;317(5838):678-81. doi: 10.1126/science.1143991. Science. 2007. PMID: 17673666 Free PMC article.
References
- Berger K H, Isberg R R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. - PubMed
- Berger K H, Merriam J J, Isberg R R. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol Microbiol. 1994;14:809–822. - PubMed
- Bonner J T. A descriptive study of the development of the slime mold Dictyostelium discoideum. Am J Bot. 1944;31:175–182.
- Brand B C, Sadosky A B, Shuman H A. The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol Microbiol. 1994;14:797–808. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources