Interleukin-7 or interleukin-15 enhances survival of Mycobacterium tuberculosis-infected mice - PubMed (original) (raw)
Interleukin-7 or interleukin-15 enhances survival of Mycobacterium tuberculosis-infected mice
M J Maeurer et al. Infect Immun. 2000 May.
Abstract
Both antigen-presenting cells and immune effector cells are required to effectively eradicate or contain Mycobacterium tuberculosis-infected cells. A variety of cytokines are involved to ensure productive "cross talk" between macrophages and T lymphocytes. For instance, infection of macrophages with mycobacteria leads to effective interleukin-7 (IL-7) and IL-15 secretion, and both cytokines are able to maintain strong cellular immune responses of alpha/beta and gamma/delta T cells. Here we show that either cytokine is able to enhance survival of M. tuberculosis-infected BALB/c mice significantly compared to application of IL-2, IL-4, or phosphate-buffered saline (as a control). Enhanced survival could be achieved only when IL-7 or IL-15 was delivered as a treatment (i.e., 3 weeks postinfection), not when it was administered at the time of infection. Increased survival of M. tuberculosis-infected animals was observed following passive transfer of spleen cells harvested from M. tuberculosis-infected, IL-7- or IL-15-treated animals, but not after transfer of spleen cells obtained from mice which received either cytokine alone. Histological examination revealed that IL-7 and IL-15 failed to significantly impact on the number and composition of granulomas formed or the bacterial load. Our data indicated that administration of IL-7 or IL-15 to M. tuberculosis-treated animals resulted in a qualitatively different cellular immune response in spleen cells as reflected by increased tumor necrosis factor alpha and decreased gamma interferon secretion in response to M. tuberculosis-infected antigen-presenting cells.
Figures
FIG. 1
Treatment schedule. (A) Mice (n = 15/group) were i.v. infected with viable bacilli. After 3 weeks, they were treated i.p. for 7 consecutive days with three 100-ng doses of IL-2, IL-4, IL-7, or IL-15 or with PBS as a control. Alternatively, mice were infected and treated concomitantly for 7 days with cytokines. The bold arrows indicate infection with M. tuberculosis. (B) Animals were either injected with PBS or infected with M. tuberculosis (M.tub.). Each group (infected or noninfected [nil]) was treated i.p. for 7 days with either IL-7 or IL-15 (three 100-ng doses/day). After 7 days, spleen cells were harvested and tested for cytokine mRNA expression, cytotoxicity, and cytokine release. Spleen cells (3 × 107) or serum (0.3 ml) from individual animals were transferred by tail vein injection to individual animals which had been preinfected 3 weeks earlier with M. tuberculosis.
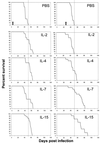
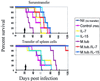
FIG. 2
Effects of IL-2, IL-4, IL-7, IL-15, and PBS on survival of _M. tuberculosis_-infected BALB/c mice. Mice were treated either at the time of infection (left panel) or 3 weeks after infection with viable bacilli (right panel). Data are from one representative experiment (n = 15 mice/group) which was performed two times. Note that IL-7 or IL-15 enhanced survival if provided as a treatment (right panel). The arrows indicate completion of the 7-day cytokine treatment (left panel, days 1 to 7; right panel, days 21 to 27). See Table 1 for associated statistical analysis data.
FIG. 3
Lack of a significant effect of IL-2, IL-4, IL-7, IL-15, or PBS on granuloma formation or liver pathology in animals killed at day 8 after infection and concomitant cytokine treatment. Formalin-fixed organs were stained with HE. Magnifications: NIL (PBS), ×680; IL-2, ×620, IL-4, ×516; IL-7, ×360; IL-15, ×564.
FIG. 4
M. tuberculosis CFU in organs of infected BALB/c mice. Mice either were not infected (Nil) or infected with M. tuberculosis and after 3 weeks were treated with cytokines for 7 days as indicated in Materials and Methods. Organs were retrieved and CFU were determined. There were 5 mice per group. Experiments were performed twice. See Table 2 for associated statistical analysis of data from one experiment.
FIG. 5
Cytokine gene expression in spleens. Mice were either noninfected (−) or infected with M. tuberculosis (+) and treated with IL-7 or IL-15. Five representative samples from individual animals are shown for each group. RNA was extracted, reverse transcribed into cDNA, and analyzed for cytokine expression by RT-PCR. Amplification of β-actin served as a positive control. Groups: A, M. tuberculosis negative, no treatment (NIL); B, M. tuberculosis positive, no treatment; C, M. tuberculosis negative, IL-7 treatment alone; D, M. tuberculosis infection plus IL-7 application; E, M. tuberculosis negative, IL-15 treatment alone; F, M. tuberculosis infection plus IL-15 treatment. No significant differences in cytokine expression could be observed within different treatment groups. In general, M. tuberculosis infection leads to decreased IL-2 and IL-4 and increased IL-10 mRNA expression.
FIG. 6
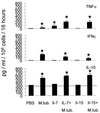
Cytokines released in response to M. tuberculosis (M.tub)-infected macrophages. Peritoneal macrophages were harvested and infected with viable M. tuberculosis bacilli and served as antigen-presenting cells. Spleen cells were obtained either from noninfected animals, or from _M. tuberculosis_-infected mice which received either PBS, IL-7, or IL-15. Spleen cells from animals which received either IL-7 or IL-15 (without M. tuberculosis infection) served as controls. Note that antigen-presenting cells alone, i.e., without responder cells, secreted up to 310 pg of IL-10/ml (horizontal bar). However, IL-10 secretion as a response to _M. tuberculosis_-positive antigen-presenting cells was observed in spleen cells harvested from animals which had been infected with M. tuberculosis, irrespective of cytokine application. IL-7 and IL-15 treatment of _M. tuberculosis_-infected animals enhanced TNF-α secretion in spleen cells. Spleen cells from _M. tuberculosis_-infected animals secreted up to 83 pg of TNF-α/ml. In contrast, spleen cells obtained from IL-7- or IL-15-treated, _M. tuberculosis_-infected animals secreted up to 200 or 180 pg TNF-α/ml, respectively. In contrast, IL-7 or IL-15 treatment appears to decrease IFN-γ secretion as a response to _M. tuberculosis_-infected stimulator cells. Spleen cells from IL-7-treated infected mice secreted up to 110 pg and those from IL-15-treated animals secreted 80 pg of IFN-γ/ml in response to _M. tuberculosis_-infected macrophages, whereas spleen cells from _M. tuberculosis_-infected animals secreted up to 200 pg of IFN-γ/ml. No IL-4 secretion could be observed (data not shown). Data for each cytokine represent mean values for five individual animals as determined by ELISA. Error bars indicate standard deviations. P values of <0.01 are indicated with stars. Levels of cytokine secretion by spleen cells obtained from different treatment groups were compared to that by cells obtained from PBS (diluent)-injected mice. Exact P values (Wilcoxon two-sample test) are given in Table 4.
FIG. 7
Passive transfer of spleen cells from M. tuberculosis (M.tub.)-infected animals treated with IL-7 or IL-15 enhances survival of animals which had been preinfected with M. tuberculosis. Animals were infected with viable bacilli and treated for 7 consecutive days with either IL-7, IL-15, or PBS. As a control, noninfected animals were treated with IL-7, IL-15, or PBS (see Fig. 1B). Spleen cells were harvested, and 3 × 107 cells from an individual animal were passively transferred via tail vein injection into an animal which had been preinfected (3 weeks earlier) with M. tuberculosis. As a control, serum from these individual treatment groups was passively transferred in _M. tuberculosis_-preinfected mice. Exclusively spleen cells harvested from animals which had been infected and treated with IL-7 or IL-15 could confer enhanced survival in preinfected mice (n = 15 mice/group). The arrows mark the points of passive transfer of serum or cells, respectively. See Table 5 for associated statistical analysis.
Similar articles
- Coadministration of interleukins 7 and 15 with bacille Calmette-Guérin mounts enduring T cell memory response against Mycobacterium tuberculosis.
Singh V, Gowthaman U, Jain S, Parihar P, Banskar S, Gupta P, Gupta UD, Agrewala JN. Singh V, et al. J Infect Dis. 2010 Aug 15;202(3):480-9. doi: 10.1086/653827. J Infect Dis. 2010. PMID: 20569158 - Suppressors of cytokine signaling inhibit effector T cell responses during Mycobacterium tuberculosis infection.
Srivastava V, Vashishta M, Gupta S, Singla R, Singla N, Behera D, Natarajan K. Srivastava V, et al. Immunol Cell Biol. 2011 Oct;89(7):786-91. doi: 10.1038/icb.2011.1. Epub 2011 May 3. Immunol Cell Biol. 2011. PMID: 21537342 - Host genetic background affects regulatory T-cell activity that influences the magnitude of cellular immune response against Mycobacterium tuberculosis.
Paula MO, Fonseca DM, Wowk PF, Gembre AF, Fedatto PF, Sérgio CA, Silva CL, Bonato VL. Paula MO, et al. Immunol Cell Biol. 2011 May;89(4):526-34. doi: 10.1038/icb.2010.116. Epub 2010 Oct 19. Immunol Cell Biol. 2011. PMID: 20956987 - [Novel vaccines against M. tuberculosis].
Okada M. Okada M. Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese. - Cytokine storm in tuberculosis and IL-6 involvement.
Boni FG, Hamdi I, Koundi LM, Shrestha K, Xie J. Boni FG, et al. Infect Genet Evol. 2022 Jan;97:105166. doi: 10.1016/j.meegid.2021.105166. Epub 2021 Nov 30. Infect Genet Evol. 2022. PMID: 34861432 Review.
Cited by
- Molecular and Functional Characterization of α Chain of Interleukin-15 Receptor (IL-15Rα) in Orange-Spotted Grouper (Epinephelus coioides) in Response to Vibrio harveyi Challenge.
Zhang Y, Wu F, Yang G, Jian J, Lu Y, Wang Z. Zhang Y, et al. Animals (Basel). 2023 Nov 24;13(23):3641. doi: 10.3390/ani13233641. Animals (Basel). 2023. PMID: 38066992 Free PMC article. - The tuberculosis vaccine candidate Bacillus Calmette-Guérin ΔureC::hly coexpressing human interleukin-7 or -18 enhances antigen-specific T cell responses in mice.
Rao M, Vogelzang A, Kaiser P, Schuerer S, Kaufmann SH, Gengenbacher M. Rao M, et al. PLoS One. 2013 Nov 13;8(11):e78966. doi: 10.1371/journal.pone.0078966. eCollection 2013. PLoS One. 2013. PMID: 24236077 Free PMC article. - Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo.
Stoklasek TA, Schluns KS, Lefrançois L. Stoklasek TA, et al. J Immunol. 2006 Nov 1;177(9):6072-80. doi: 10.4049/jimmunol.177.9.6072. J Immunol. 2006. PMID: 17056533 Free PMC article. - Interleukin-15 as an immune adjuvant to increase the efficacy of Mycobacterium bovis bacillus Calmette-Guérin vaccination.
Umemura M, Nishimura H, Saito K, Yajima T, Matsuzaki G, Mizuno S, Sugawara I, Yoshikai Y. Umemura M, et al. Infect Immun. 2003 Oct;71(10):6045-8. doi: 10.1128/IAI.71.10.6045-6048.2003. Infect Immun. 2003. PMID: 14500526 Free PMC article.
References
- Arachi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1. - PubMed
- Bamford R N, Battiata A P, Waldmann T A. IL-15: the role of translational regulation in their expression. J Leukoc Biol. 1996;59:476–480. - PubMed
- Beckman E M, Melian A, Behar S M, Sieling P A, Chatterjee D, Furlong S T, Matsumoto R, Rosat J P, Modlin R L, Porcelli S A. CD1c restricts responses of mycobacteria-specific T cells. Evidence for antigen presentation by a second member of the human CD1 family. J Immunol. 1996;157:2795–2803. - PubMed
- Beckman E M, Porcelli S A, Morita C T, Behar S M, Furlong S T, Brenner M B. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature. 1994;372:691–694. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical