The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae - PubMed (original) (raw)
The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae
A Wiederkehr et al. J Cell Biol. 2000.
Abstract
In Saccharomyces cerevisiae, endocytic material is transported through different membrane-bound compartments before it reaches the vacuole. In a screen for mutants that affect membrane trafficking along the endocytic pathway, we have identified a novel mutant disrupted for the gene YJL204c that we have renamed RCY1 (recycling 1). Deletion of RCY1 leads to an early block in the endocytic pathway before the intersection with the vacuolar protein sorting pathway. Mutation of RCY1 leads to the accumulation of an enlarged compartment that contains the t-SNARE Tlg1p and lies close to areas of cell expansion. In addition, endocytic markers such as Ste2p and the fluorescent dyes, Lucifer yellow and FM4-64, were found in a similar enlarged compartment after their internalization. To determine whether rcy1Delta is defective for recycling, we have developed an assay that measures the recycling of previously internalized FM4-64. This method enables us to follow the recycling pathway in yeast in real time. Using this assay, it could be demonstrated that recycling of membranes is rapid in S. cerevisiae and that a major fraction of internalized FM4-64 is secreted back into the medium within a few minutes. The rcy1Delta mutant is strongly defective in recycling.
Figures
Figure 9
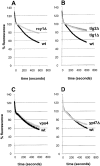
FM4-64 recycling is inhibited in _rcy1_Δ, _tlg1_Δ, and _tlg2_Δ cells. All FM4-64 recycling measurements were done as described in Fig. 7. (A and B) Recycling of FM4-64 at 24°C is strongly inhibited in _rcy1_Δ (A, grey line), _tlg1_Δ (B, grey line), and _tlg2_Δ (B, dark grey line) compared with their corresponding wild-type (black lines) cells. vps4 (C, grey line) and ypt7 (D, grey line) recycle FM4-64 at similar rates as the corresponding wild-type cells (black lines).
Figure 1
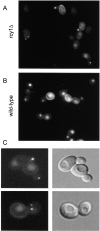
Accumulation of LY in punctuate nonvacuolar structures close to and in the bud of the _rcy1_-null mutant cells. rcy1 mutant (A and C) and wild-type cells (B) were incubated in YPUAD containing LY (4 mg/ml) for 1 h at 24°C. The cells were then washed and visualized using fluorescence optics. (C) rcy1 mutant cells visualized at higher magnification using either fluorescence (left panels) or Nomarski optics (right panels).
Figure 2
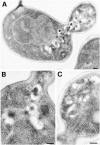
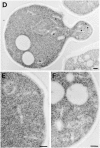
Tlg1p localizes to the enlarged compartment of the _rcy1_Δ mutant. Logarithmically growing cells were fixed, dehydrated, and embedded for EM as described in Materials and Methods. Cell sections were then incubated with purified anti-Tlg1p antibodies followed by anti-IgG secondary antibodies. The gold-coupled secondary antibodies can be seen on the sections as black dots with a diameter of 10 nm. Immunogold-labeled sections of _rcy1_Δ (A–C) and the corresponding wild-type (D–F) are shown. Bars, 200 nm. (D) Tlg1p-positive membranes are indicated by a star.
Figure 2
Tlg1p localizes to the enlarged compartment of the _rcy1_Δ mutant. Logarithmically growing cells were fixed, dehydrated, and embedded for EM as described in Materials and Methods. Cell sections were then incubated with purified anti-Tlg1p antibodies followed by anti-IgG secondary antibodies. The gold-coupled secondary antibodies can be seen on the sections as black dots with a diameter of 10 nm. Immunogold-labeled sections of _rcy1_Δ (A–C) and the corresponding wild-type (D–F) are shown. Bars, 200 nm. (D) Tlg1p-positive membranes are indicated by a star.
Figure 3
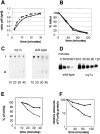
Degradation of α-factor pheromone and the plasma membrane protein uracil permease is defective in the _rcy1_Δ mutant cells. Internalization (A and B) and degradation (C–F) of α-factor (A, C, and E) and uracil permease (B, D, and F) was studied. (A) Internalization of α-factor was followed as the ratio of internalized radio-labeled α-factor (resistant to pH 1.1 wash) divided by the total cell-associated cpm (internalized plus surface receptor-bound α-factor retained after pH 6 wash). _rcy1_Δ cells (solid line) internalized [S35]α-factor at rates similar to wild-type cells (dashed line). (B) Transport of [3H]uracil into yeast cells was used as a measure for the relative amounts of uracil permease exposed at the cell surface. Upon addition of cycloheximide (100 μg/ml), uracil permease levels at the cell surface decreased. Uracil uptake is plotted as the percentage activity at the initial timepoint. The decrease of uracil transport over time is identical in the _rcy1_Δ (solid line) and wild-type cells (dashed line). (C) Degradation of α-factor. Cells were allowed to take up radiolabeled α-factor as described in A. Membrane traffic was stopped and cells were washed in cold buffer, pH 1.1, at the indicated timepoints in minutes. Cells were extracted and cell-associated radioactivity was analyzed by TLC and autoradiography. The disappearance of intact (i) α-factor was quantified (E). In wild-type cells, degraded α-factor (d) was already observed 20 min after internalization at 24°C had been initiated. (D) Degradation of uracil permease. At the indicated times after addition of cycloheximide (100 μg/ml), total proteins were extracted. The intensity of the uracil permease band as detected on the Western blot was quantified and the values presented as the relative amounts of the signal before adding cycloheximide (F). (E and F) Wild-type (dashed line) and _rcy1_Δ (solid line).
Figure 4
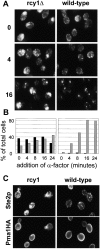
Ste2p traffic is blocked in an endocytic compartment. (A) Ligand-induced endocytosis of Ste2p was followed over time. _rcy1_Δ (left column) and wild-type cells (right column) were exposed to α-factor (10−7 M) for the indicated times in minutes. Cells were fixed and stained using a polyclonal antibody against Ste2p. Note the large Ste2p-positive membranes in the _rcy1_Δ strain. (B) For comparison of _rcy1_Δ (left) and wild-type strains (right) the cells were quantified for the presence of Ste2p-positive compartments. The sizes of Ste2p-positive compartments were judged by immunofluorescence (not real size of compartments). The percentage of total cells with Ste2p-positive structures with an apparent size >1 μm at its largest diameter is represented by the black bars. The grey bars show the percentage of cells with smaller round Ste2p-positive compartments of ∼0.5–1 μm in diameter. (C) Pma1p does not colocalize to the internal Ste2p-positive membranes. A wild-type strain (right panels) and the _rcy1_Δ mutant strain (left panels) expressing a genomic hemagglutinin-tagged version of Pma1p were labeled for Ste2p as in A (top panels) and Pma1HA using a monoclonal anti-hemagglutinin (bottom panels) by double immunofluorescence. No cross-bleeding was observed when either of the two primary antibodies was omitted (not shown).
Figure 5
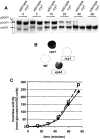
CPY targeting and invertase secretion are normal in the _rcy1_Δ mutant. (A) Maturation of CPY occurs at a normal rate in _rcy1_Δ mutant cells. Wild-type (left lanes) and _rcy1_Δ (right lanes) cells were pulsed for 4 min with 150 μCi/ml of [35S]methionine/cysteine labeling mixture and chased for the indicated times (see Materials and Methods). CPY was immunoprecipitated from total protein extracts. The ER form of CPY (p1CPY) and the Golgi form of CPY (p2CPY) disappear in both strains at approximately equal rates. (B) Like wild-type cells (WT), _rcy1_Δ cells do not secrete CPY. Secretion of CPY was assayed on nitrocellulose filters. vps mutants secrete a fraction of CPY, which binds to nitrocellulose filters and can be detected by probing with a CPY-specific antibody. Due to inefficient sorting of CPY in the vps mutant (vps1) and the class E vps mutant (vps4), a fraction of CPY is secreted, which results in a strong signal on the membrane. (C) Invertase secretion occurs at normal rates from _rcy1_Δ cells. Cells grown in YPUAD (2% glucose) were washed and resuspended in a medium containing 2% sucrose and 0.05% glucose at time 0. Samples were taken at different timepoints after this induction and assayed for secreted invertase activity (see Materials and Methods). Both the onset and the extent of invertase secretion from _rcy1_Δ cells (solid line) and wild-type cells (dashed line) were similar.
Figure 6
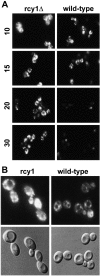
Differences of FM4-64 traffic in _rcy1_Δ (left) and wild-type (right) strains. (A) Cells were incubated for 10 min in YPUAD in the presence of 20 μM FM4-64. The cells were then washed in fresh medium to remove the surface-bound dye and further incubated. Samples were taken and stopped in cold buffer containing 10 mM NaN3 and 10 mM NaF. The times on the left of the figure indicate the minutes after addition of the dye. The cells were visualized using fluorescence optics. (B) For comparison of vacuolar FM4-64 staining (top), Nomarski images (bottom) are shown for the 20-min timepoint.
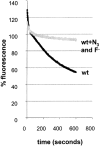
Figure 7
Measuring recycling in yeast. Cells were allowed to take up FM4-64 (40 μM) for 12 min as described in Fig. 6. The cells were then washed in ice-cold SD minimal medium. Thoroughly washed cells were resuspended in SD medium at 24°C. FM4-64 fluorescence in the cell suspension was measured over time in a spectrofluorometer (see Materials and Methods). (A) Fluorescence associated with wild-type cells (black line) decreases over time. This decrease was blocked when the cells were resuspended in SD medium containing NaN3 and NaF (grey line).
Figure 8
FM4-64 is recycled into the medium in a _SEC18_-dependent manner. (A) Wild-type cells (grey bars) or _rcy1_Δ cells (black bars) were loaded with FM4-64 at 24°C, washed, and incubated for the indicated time. Relative fluorescence changes seen after addition of 1% CHAPS were quantified. The fluorescence change seen after 8 min with wild-type cells was set to 100%. (B) Aliquots of recycling cells prepared as in A were treated with (+) or without (−) 1% CHAPS for 1 min. Then, 10 mM sodium azide and 10 mM sodium fluoride were added. After centrifugation, the fluorescence inside the cells was measured and divided by the cell number. After resuspension of cells that did not receive CHAPS (−) in fresh medium, 1% CHAPS was added (−/+). No increase in total fluorescence was observed upon addition of CHAPS under these conditions. In agreement with continuous measurements (Fig. 7), the cell pellet analyzed at 2 min contained about twice as much fluorescence as the cells that had recycled for 8 min. The fluorescence in the pellet of wild-type cells at 8 min was set to 100%. (C and D) Wild-type (black lines) and sec18-1 (grey lines) mutant cells were resuspended in SD medium prewarmed to 23°C (B) or 36°C (C). At 36°C, the recycling of FM4-64 was strongly inhibited in the sec18-1 strain compared with the wild-type. (E) Quantitation of the fluorescence changes in response to 1% CHAPS of the different mutants used in this study. The average of two independent experiments is shown for each mutant and condition. The fluorescence change due to addition of CHAPS seen after 8 min with wild-type cells was set to 100%. Standard error bars are indicated where more than two independent experiments have been performed.
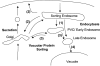
Figure 10
Model for membrane traffic in the vacuolar system. (1) Traffic from the postulated sorting endosome to the PVC is inhibited in the _rcy1_Δ mutant. (2) Two possible pathways that contribute to recycling from the sorting endosome to the plasma membrane, one passing through the Golgi and the other directly to the plasma membrane. (3) Traffic steps blocked in class E vps mutants, which do not affect recycling. (4) Traffic from the late endosome to the vacuole, which is also not required for recycling.
Similar articles
- Identification and characterization of Saccharomyces cerevisiae mutants defective in fluid-phase endocytosis.
Wiederkehr A, Meier KD, Riezman H. Wiederkehr A, et al. Yeast. 2001 Jun;18(8):759-73. doi: 10.1002/yea.726. Yeast. 2001. PMID: 11378903 - Endocytic recycling in yeast is regulated by putative phospholipid translocases and the Ypt31p/32p-Rcy1p pathway.
Furuta N, Fujimura-Kamada K, Saito K, Yamamoto T, Tanaka K. Furuta N, et al. Mol Biol Cell. 2007 Jan;18(1):295-312. doi: 10.1091/mbc.e06-05-0461. Epub 2006 Nov 8. Mol Biol Cell. 2007. PMID: 17093059 Free PMC article. - Soi3p/Rav1p functions at the early endosome to regulate endocytic trafficking to the vacuole and localization of trans-Golgi network transmembrane proteins.
Sipos G, Brickner JH, Brace EJ, Chen L, Rambourg A, Kepes F, Fuller RS. Sipos G, et al. Mol Biol Cell. 2004 Jul;15(7):3196-209. doi: 10.1091/mbc.e03-10-0755. Epub 2004 Apr 16. Mol Biol Cell. 2004. PMID: 15090613 Free PMC article. - Novel pathways, membrane coats and PI kinase regulation in yeast lysosomal trafficking.
Burd CG, Babst M, Emr SD. Burd CG, et al. Semin Cell Dev Biol. 1998 Oct;9(5):527-33. doi: 10.1006/scdb.1998.0255. Semin Cell Dev Biol. 1998. PMID: 9835640 Review. - The yeast endocytic membrane transport system.
Munn AL. Munn AL. Microsc Res Tech. 2000 Dec 15;51(6):547-62. doi: 10.1002/1097-0029(20001215)51:6<547::AID-JEMT5>3.0.CO;2-D. Microsc Res Tech. 2000. PMID: 11169857 Review.
Cited by
- Multiple functions of sterols in yeast endocytosis.
Heese-Peck A, Pichler H, Zanolari B, Watanabe R, Daum G, Riezman H. Heese-Peck A, et al. Mol Biol Cell. 2002 Aug;13(8):2664-80. doi: 10.1091/mbc.e02-04-0186. Mol Biol Cell. 2002. PMID: 12181337 Free PMC article. - Retrograde trafficking and quality control of yeast synaptobrevin, Snc1, are conferred by its transmembrane domain.
Ma M, Burd CG. Ma M, et al. Mol Biol Cell. 2019 Jul 1;30(14):1729-1742. doi: 10.1091/mbc.E19-02-0117. Epub 2019 May 8. Mol Biol Cell. 2019. PMID: 31067149 Free PMC article. - Budding Yeast Has a Minimal Endomembrane System.
Day KJ, Casler JC, Glick BS. Day KJ, et al. Dev Cell. 2018 Jan 8;44(1):56-72.e4. doi: 10.1016/j.devcel.2017.12.014. Epub 2018 Jan 8. Dev Cell. 2018. PMID: 29316441 Free PMC article. - Requirements of Slm proteins for proper eisosome organization, endocytic trafficking and recycling in the yeast Saccharomyces cerevisiae.
Kamble C, Jain S, Murphy E, Kim K. Kamble C, et al. J Biosci. 2011 Mar;36(1):79-96. doi: 10.1007/s12038-011-9018-0. J Biosci. 2011. PMID: 21451250 - COPI mediates recycling of an exocytic SNARE by recognition of a ubiquitin sorting signal.
Xu P, Hankins HM, MacDonald C, Erlinger SJ, Frazier MN, Diab NS, Piper RC, Jackson LP, MacGurn JA, Graham TR. Xu P, et al. Elife. 2017 Oct 23;6:e28342. doi: 10.7554/eLife.28342. Elife. 2017. PMID: 29058666 Free PMC article.
References
- Bai C., Sen P., Hofmann K., Ma L., Goebl M., Harper J.W., Elledge S.J. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. - PubMed
- Benito B., Moreno E., Lagunas R. Half-life of the plasma membrane ATPase and its activating system in resting yeast cells. Biochim. Biophys. Acta. 1991;1063:265–268. - PubMed
- Betz W.J., Bewick G.S. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992;255:200–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases