An in vivo function for the transforming Myc protein: elicitation of the angiogenic phenotype - PubMed (original) (raw)
An in vivo function for the transforming Myc protein: elicitation of the angiogenic phenotype
C V Ngo et al. Cell Growth Differ. 2000 Apr.
Abstract
The ability of neoplastic cells to recruit blood vasculature is crucial to their survival in the host organism. However, the evidence linking dominant oncogenes to the angiogenic switch remains incomplete. We demonstrate here that Myc, an oncoprotein implicated in many human malignancies, stimulates neovascularization. As an experimental model, we used Rat-1A fibroblasts that form vascular tumors upon transformation by Myc in immunocompromised mice. Our previous work and the use of neutralizing antibodies reveal that in these cells, the angiogenic switch is achieved via down-modulation of thrombospondin-1, a secreted inhibitor of angiogenesis, whereas the levels of vascular endothelial growth factor, a major activator of angiogenesis, remain high and unaffected by Myc. Consistent with this finding, overexpression of Myc confers upon the conditioned media the ability to promote migration of adjacent endothelial cells in vitro and corneal neovascularization in vivo. Furthermore, mobilization of estrogen-dependent Myc in vivo with the appropriate steroid provokes neovascularization of cell implants embedded in Matrigel. These data suggest that Myc is fully competent to trigger the angiogenic switch in vivo and that secondary events may not be required for neovascularization of Myc-induced tumors.
Figures
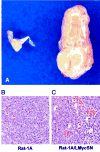
Fig. 1
Gross appearance and histological examination of tumors formed by Rat-1A/LMycSN cells. A, the s.c. tumor formed by Rat-1A/LMycSN cells 10 days after injection into a Rag-1−/− mouse (right specimen). The cell mass representing parental Rat-1A cells is shown on the left. B and C, respectively, histological staining of the above-mentioned specimens with H&E. The_white arrows_ in C point at blood vessels.
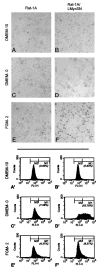
Fig. 2
Analysis of cell viability on culturing in different media.A–F, phase-contrast microphotographs of Rat-1A and Rat-1A/LMycSN cells incubated in DMEM supplemented with 10% (DMEM-10) or 0% FCS (DMEM-0) or in FGM-2._A_′_–F_′, flow cytometry profiles of the same cultures on fluorescent staining for DNA breaks. M1 and M2 populations refer to apoptotic and viable cells, respectively; _numbers below M1_refer to the percentages of apoptotic cells.
Fig. 3
Stimulation of corneal neovascularization by CM from Myc-expressing cells.Left column, phase-contrast microphotographs of dissected corneas; right column, fluorescence by FITC-dextran injected i.v. and marking perfused blood vessels. Sponges containing CM from Rat-1A and Rat-1A/LMycSN cells were implanted in two animals (mouse #1 and mouse #2) and elicited consistent responses (negative in the case of Rat-1A cells and positive in the case of Rat-1A/LMycSN cells). A total number of six sponges were analyzed for each CM in two independent experiments yielding similar results. bFGF was used as a control proangiogenic molecule.
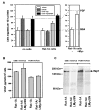
Fig. 4
The effect of Myc on the expression of pro- and antiangiogenic factors in Rat-1A fibroblasts. A, endothelial cell chemotaxis assay performed with CM from Rat-1A and Rat-1A/LMycSN cells, as described in “Materials and Methods.” CM were used at a total protein concentration of 20 μg/ml. Supplemental reagents included the anti-Tsp-1 monoclonal antibody A4.1 (20 μg/ml) and bFGF (10 ng/ml). Serum-free DMEM supplemented with BSA was used as a negative control. Dotted line labeled BSA, background migration toward media containing BSA alone. Dotted line labeled bFGF, migration toward media containing both BSA and bFGF, a strong angiogenic factor. B, murine VEGF ELISA performed on media conditioned by Rat-1A (bars 1 and ) and Rat-1A/LMycSN (bars 2 and ) cells cultured in either complete (bars 1 and ) or serum-free (bars 3 and ) media. C, radioimmunoprecipitation of the Tsp-1 protein from media conditioned by Rat-1A (Lanes 1 and ) and Rat-1A/LMycSN (Lanes 2 and ) cells cultured in either complete (Lanes 1 and ) or serum-free (Lanes 3 and ) media. Migration of MultiMark Multi-Colored Protein Standards (Novel Experimental Technologies, San Diego, CA) is indicated on the_left_. Due to extensive glycosylation, Tsp-1 has an apparent molecular mass of approximately 170 kDa, as indicated by the_arrow_ on the right.
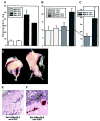
Fig. 5
Neovascularization of Matrigel implants containing Myc-expressing Rat-1 cells. Cells tested were expressing either constitutively active Myc (A) or the MycER derivative (B–F). A–C, the hemoglobin contents of implants from vehicle (−OHT)- and OHT (+OHT)-treated animals. _MG_refers to empty Matrigels, and Rat and _Myc_refer to Matrigels containing parental and LMycSN-infected Rat-1A cells, respectively. MERmass and MER-9 refer, respectively, to Matrigels containing mass cultures and a single cell clone of BabePuroMycER-infected Rat1A-cells. Each experiment was repeated at least twice, yielding similar results. Each bar, except for that of Matrigel alone, represents data obtained from at least four animals (2 pellets/animal). In the case of Rat-1A/MycER-9 cells, a total of 12 animals were analyzed. The variability in the experiment with 4-OHT-treated LMycSN Matrigels was negligible, resulting in an invisible error bar.D, gross appearance of Matrigels containing uninduced (left) and 4-OHT-induced (right) Rat-1A/My-cER-9 cells. E and F, histological staining of the two Matrigels shown in D(left and right, respectively).Small white arrows point at blood vessels in the surrounding connective tissue. Large white arrows point at blood vessels traversing the Matrigel.
References
- Bouchard C, Staller P, Eilers M. Control of cell proliferation by Myc. Trends Cell Biol. 1998;8:202–206. - PubMed
- Zornig M, Evan GI. Cell cycle: on target with Myc. Curr Biol. 1996;6:1553–1556. - PubMed
- Grandori C, Eisenman RN. Myc target genes. Trends Biochem Sci. 1997;22:177–181. - PubMed
- Campisi J, Gray HE, Pardee AB, Dean M, Sonenshein GE. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984;36:241–247. - PubMed
- Kelly K, Cochran BH, Stiles CD, Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983;35:603–610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials