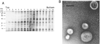Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles - PubMed (original) (raw)
Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles
A L Horstman et al. J Biol Chem. 2000.
Abstract
Escherichia coli and other Gram-negative bacteria produce outer membrane vesicles during normal growth. Vesicles may contribute to bacterial pathogenicity by serving as vehicles for toxins to encounter host cells. Enterotoxigenic E. coli (ETEC) vesicles were isolated from culture supernatants and purified on velocity gradients, thereby removing any soluble proteins and contaminants from the crude preparation. Vesicle protein profiles were similar but not identical to outer membranes and differed between strains. Most vesicle proteins were resistant to dissociation, suggesting they were integral or internal. Thin layer chromatography revealed that major outer membrane lipid components are present in vesicles. Cytoplasmic membranes and cytosol were absent in vesicles; however, alkaline phosphatase and AcrA, periplasmic residents, were localized to vesicles. In addition, physiologically active heat-labile enterotoxin (LT) was associated with ETEC vesicles. LT activity correlated directly with the gradient peak of vesicles, suggesting specific association, but could be removed from vesicles under dissociating conditions. Further analysis revealed that LT is enriched in vesicles and is located both inside and on the exterior of vesicles. The distinct protein composition of ETEC vesicles and their ability to carry toxin may contribute to the pathogenicity of ETEC strains.
Figures
Fig. 1. Velocity gradient centrifugation separates vesicles from loosely associated proteins
A, ETEC 2 vesicles (140 µg) were loaded on the bottom of an Optiprep gradient. After centrifugation, equal volumes (20 µl) of 750-µl fractions were applied to 12.5% SDS-PAGE and silver-stained. B, electron micrograph of ETEC 2 vesicles after gradient purification. Vesicles were visualized by negative staining and measured. Bar = 100 nm.
Fig. 2. Comparison of HB101 and ETEC 2 outer membranes and vesicles: variation with strain and growth conditions
Outer membranes (OM) and vesicles (Ves) (0.5 µg) were applied to 12.5% SDS-PAGE and silver-stained. OMPs F, C, and A are well established, abundant proteins in the outer membrane. OmpX and OmpW were identified by N-terminal sequencing.
Fig. 3. Thin layer chromatography of HB101 and ETEC 2 outer membranes (OM) and vesicles (Ves)
Glycerophospholipid and LPS analyses were performed on outer membrane and vesicle preparations (10 µg of protein). PE, phosphatidylethanolamine; PG, phosphatidylglycerol; CL, cardiolipin; *, modified lipid A.
Fig. 4
A, vesicle proteins are not extensively dissociated by ionic disruption or urea. Vesicle protein (1 µg) was treated for 60 min at 37 °C (unless specified) in equal volumes (20 µl) containing 50 m
m
HEPES, pH 6.8, 0.5
m
NaCl, 0.1
m
Na2CO3, pH 11, 0.8
m
urea, or 1% SDS. Samples were centrifuged (30 min, 40,000 × g or 150,000 × g, as indicated), the supernatants were removed, and pellets were resuspended in equal volumes (20 µl). The pellets (P) and supernatants (S) were applied to 17% SDS-PAGE and silver-stained. B, LT activity is present in both pellets and supernatants after dissociation treatment. Pellets (black bars) and supernatants (gray bars) from samples described in panel A were applied to Y1 cells (1.25 µg/well). Error bars = ± 1 S.E., n = 3.
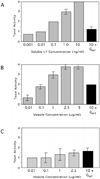
Fig. 5. Soluble and vesicle-associated LT induces morphological changes in Y1 cells that are inhibitable by GM1
Y1 adrenal cell morphology was scored after incubation with soluble LT (A), ETEC 2 vesicles (B), or HB101 vesicles (C). To demonstrate LT-specific activity, GM1 (100 ng) was preincubated with LT and vesicles (black bars). Buffer alone resulted in a morphological score of 0 in all experiments. Error bars = ± 1 S.E., n = 3.
Fig. 6. GM1-inhibitable LT activity comigrates with the ETEC 2 vesicle peak on an Optiprep gradient
A, equal volumes (100 µl) of Optiprep gradient fractions (approximately 5 µg/ml in the peak fraction) were incubated with Y1 adrenal cells and scored after 18 h. To demonstrate LT-specific activity, GM1 (100 ng) was preincubated with fractions (black bars). Optiprep alone resulted in a morphological score of 0. _Error bars_=±1 S.E. B, fractions (20 µl) analyzed in panel A were applied to 12.5% SDS-PAGE and silver-stained.
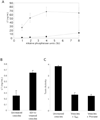
Fig. 7. LT enrichment and localization
A, ETEC 2 vesicles (triangles) and periplasm (squares) containing equal amounts of alkaline phosphatase activity were subjected to the LT ELISA. HB101 vesicles (8 sigma units (SU)) yielded an absorbance corresponding to one-fourth that of ETEC 2 vesicles (8 sigma units). B, intact and 0.1
m
EDTA-disrupted vesicles (10 µg) were applied to the LT ELISA. The presence of EDTA did not affect the soluble LT standard curve. C, ETEC 2 vesicles (2.5 µg/ml), untreated, preincubated with GM1, and digested with Pronase, were applied to the Y1 cell assay, and the morphology was scored, blinded. HB101 vesicles demonstrated normal background activity. Error bars = ± 1 S.E., n = 3.
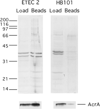
Fig. 8. Affinity binding of intact ETEC vesicles via exterior LT
ETEC 2 and HB101 (40 µg) vesicles were incubated with Sepharose beads. A portion of the load (40 ng) and one-half of the Sepharose-bound proteins were applied to SDS-PAGE and either Coomassie-stained (upper) or transferred to PVDF and immunoblotted using anti-AcrA antibody (lower).
Similar articles
- The release of outer membrane vesicles from the strains of enterotoxigenic Escherichia coli.
Wai SN, Takade A, Amako K. Wai SN, et al. Microbiol Immunol. 1995;39(7):451-6. doi: 10.1111/j.1348-0421.1995.tb02228.x. Microbiol Immunol. 1995. PMID: 8569529 - Porcine Enterotoxigenic Escherichia coli Strains Differ in Their Capacity To Secrete Enterotoxins through Varying YghG Levels.
Wang H, Sanz Garcia R, Cox E, Devriendt B. Wang H, et al. Appl Environ Microbiol. 2020 Nov 24;86(24):e00523-20. doi: 10.1128/AEM.00523-20. Print 2020 Nov 24. Appl Environ Microbiol. 2020. PMID: 32561576 Free PMC article. - Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells.
Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. Kesty NC, et al. EMBO J. 2004 Nov 24;23(23):4538-49. doi: 10.1038/sj.emboj.7600471. Epub 2004 Nov 18. EMBO J. 2004. PMID: 15549136 Free PMC article. - Context-dependent activation kinetics elicited by soluble versus outer membrane vesicle-associated heat-labile enterotoxin.
Chutkan H, Kuehn MJ. Chutkan H, et al. Infect Immun. 2011 Sep;79(9):3760-9. doi: 10.1128/IAI.05336-11. Epub 2011 Jun 27. Infect Immun. 2011. PMID: 21708992 Free PMC article. - Adhesion of enterotoxigenic Escherichia coli in humans and animals.
Levine MM. Levine MM. Ciba Found Symp. 1981;80:142-60. doi: 10.1002/9780470720639.ch10. Ciba Found Symp. 1981. PMID: 6114818 Review.
Cited by
- The extracellular RNA complement of Escherichia coli.
Ghosal A, Upadhyaya BB, Fritz JV, Heintz-Buschart A, Desai MS, Yusuf D, Huang D, Baumuratov A, Wang K, Galas D, Wilmes P. Ghosal A, et al. Microbiologyopen. 2015 Apr;4(2):252-266. doi: 10.1002/mbo3.235. Epub 2015 Jan 21. Microbiologyopen. 2015. PMID: 25611733 Free PMC article. - Identification of a protein secretory pathway for the secretion of heat-labile enterotoxin by an enterotoxigenic strain of Escherichia coli.
Tauschek M, Gorrell RJ, Strugnell RA, Robins-Browne RM. Tauschek M, et al. Proc Natl Acad Sci U S A. 2002 May 14;99(10):7066-71. doi: 10.1073/pnas.092152899. Proc Natl Acad Sci U S A. 2002. PMID: 12011463 Free PMC article. - Biochemical and functional characterization of Helicobacter pylori vesicles.
Olofsson A, Vallström A, Petzold K, Tegtmeyer N, Schleucher J, Carlsson S, Haas R, Backert S, Wai SN, Gröbner G, Arnqvist A. Olofsson A, et al. Mol Microbiol. 2010 Sep;77(6):1539-55. doi: 10.1111/j.1365-2958.2010.07307.x. Epub 2010 Aug 5. Mol Microbiol. 2010. PMID: 20659286 Free PMC article. - Murine Norovirus Interaction with Enterobacter cloacae Leads to Changes in Membrane Stability and Packaging of Lipid and Metabolite Vesicle Content.
Mosby CA, Edelmann MJ, Jones MK. Mosby CA, et al. Microbiol Spectr. 2023 Mar 21;11(2):e0469122. doi: 10.1128/spectrum.04691-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36943087 Free PMC article. - [Research progress on the role of extracellular vesicles in bacterial pathogenesis].
Guo S, Zhao L, Tao S, Zhang C. Guo S, et al. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018 Dec 15;32(12):1597-1604. doi: 10.7507/1002-1892.201805075. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018. PMID: 30569690 Free PMC article. Review. Chinese.
References
- Wensink J, Gankema H, Jansen WH, Guinee PA, Witholt B. Biochim. Biophys. Acta. 1978;514:128–136. - PubMed
- Donta ST, Moon HW, Whipp SC. Science. 1974;183:334–336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources