The extracellular signal-regulated kinase cascade is required for NMDA receptor-independent LTP in area CA1 but not area CA3 of the hippocampus - PubMed (original) (raw)
The extracellular signal-regulated kinase cascade is required for NMDA receptor-independent LTP in area CA1 but not area CA3 of the hippocampus
B I Kanterewicz et al. J Neurosci. 2000.
Abstract
Activation of extracellular signal-regulated kinase (ERK) has been shown to be necessary for NMDA receptor-dependent long-term potentiation (LTP). We studied the role of ERK in three forms of NMDA receptor-independent LTP: LTP induced by very high-frequency stimulation (200 Hz-LTP), LTP induced by the K(+) channel blocker tetraethylammonium (TEA) (TEA-LTP), and mossy fiber (MF) LTP (MF-LTP). We found that ERK was activated in area CA1 after the induction of both 200 Hz-LTP and TEA-LTP and that this activation required the influx of Ca(2+) through voltage-gated Ca(2+) channels. Inhibition of the ERK signaling cascade with either PD 098059 or U0126 prevented the induction of both 200 Hz-LTP and TEA-LTP in area CA1. In contrast, neither PD 098059 nor U0126 prevented MF-LTP in area CA3 induced by either brief or long trains of high-frequency stimulation. U0126 also did not prevent forskolin-induced potentiation in area CA3. However, incubation of slices with forskolin, an activator of the cAMP-dependent protein kinase (PKA) cascade, did result in increases in active ERK and cAMP response element-binding protein (CREB) phosphorylation in area CA3. The forskolin-induced increase in active ERK was inhibited by U0126, whereas the increase in CREB phosphorylation was not, which suggests that in area CA3 the PKA cascade is not coupled to CREB phosphorylation via ERK. Overall, our observations indicate that activation of the ERK signaling cascade is necessary for NMDA receptor-independent LTP in area CA1 but not in area CA3 and suggest a divergence in the signaling cascades underlying NMDA receptor-independent LTP in these hippocampal subregions.
Figures
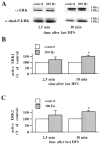
Fig. 1.
200 Hz-LTP is associated with an increase in active ERK1/ERK2. A, Representative ERK Western blots of area CA1 subregions from control slices and slices given three 1 sec trains of HFS (200 Hz). The slices given 200 Hz HFS were analyzed either 2.5 or 10 min after the final train of HFS and compared with control slices from the same recording chamber. The α-ERK antibody detects ERK1 and ERK2 independent of phosphorylation state. The α-dual-P-ERK antibody detects the dually phosphorylated, active forms of ERK1 and ERK2. B, Normalized active ERK1 immunoreactivity 2.5 min (n = 6) and 10 min (n = 4) after the final train of HFS.C, Normalized active ERK2 immunoreactivity 2.5 min (n = 6) and 10 min (n = 4) after the final train of HFS. Error bars in B and_C_ indicate SEM; * denotes statistical significance compared with control (p < 0.05 by paired Student's t test).
Fig. 2.
Nifedipine blocks the increase in active ERK1/ERK2 associated with 200 Hz-LTP. A, Representative Western blot of the area CA1 subregion from a control slice and a slice given the 200 Hz-LTP-inducing HFS in the presence of 10 μ
m
nifedipine. The slice was analyzed 10 min after the final train of HFS.B, Normalized active ERK1/ERK2 immunoreactivity 10 min after the delivery of 200 Hz-LTP-inducing HFS (n = 4). Error bars indicate SEM.
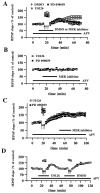
Fig. 3.
Effect of U0126 and PD 098059 on 200 Hz-LTP and NMDA receptor-independent synaptic transmission. A, Blockade of 200 Hz-LTP by either U0126 or PD 098059.Open circles are ensemble averages of the fEPSP slope from slices given 200 Hz-LTP-inducing HFS (indicated by the_arrows_) in the presence of 0.33% DMSO (n = 6). Open _squares_are ensemble averages of the fEPSP slope from slices given 200 Hz-LTP-inducing HFS in the presence of 20 μ
m
U0126 (n = 6). Closed squares are ensemble averages from slices given 200 Hz-LTP-inducing HFS in the presence of 50 μ
m
PD 098059 (n = 6). DMSO, U0126, or PD 098059 was present in the perfusing solution 7.5 min before, during, and 7.5 min after the delivery of the HFS (total time in the solution was 20 min, indicated by the horizontal bar). Both MEK inhibitors significantly blocked LTP 45 min after the final train of HFS (p < 0.001 by paired Student's_t_ test). B, Effect of MEK inhibitors on NMDA receptor-independent synaptic transmission. Baseline responses were recorded for 15 min before the slices were perfused for 20 min (indicated by the horizontal bar) with either 20 μ
m
U0126 (open squares; n = 4) or 50 μ
m
PD 098059 (closed squares; n = 4). Responses were recorded for an additional 30 min after the washout of each compound.C, Effect of MEK inhibitors on established 200 Hz-LTP. Baseline responses were recorded for 17.5 min before the delivery of HFS (indicated by the_arrows_). Twenty-five minutes after the delivery of HFS, slices were perfused with either 20 μ
m
U0126 (open squares; n = 4) or 50 μ
m
PD 098059 (closed squares; n = 4) for 20 min. Responses were recorded for 30 min after the washout of each compound.D, Induction of 200 Hz-LTP after the washout of U0126. Delivery of 200 Hz-LTP-inducing HFS (indicated by the_arrows_ on the left) in the presence of 20 μ
m
U0126 (indicated by the horizontal bar) resulted in the blockade of LTP. Forty minutes after the washout of U0126, 200 Hz-LTP-inducing HFS (indicated by the_arrows_ on the right) was delivered in the presence of 0.33% DMS0 (indicated by the horizontal bar), resulting in LTP. The NMDA receptor antagonist APV (50 μ
m
) was present in the perfusing solution (indicated by the horizontal bar) in all experiments in A–D. Error bars in all _panels_indicate SEM.
Fig. 4.
TEA-LTP is associated with an increase in active ERK1/ERK2. A, Representative ERK Western blots of area CA1 subregions from control slices and slices exposed to 25 m
m
TEA for 10 min. The slices exposed to TEA were analyzed either 2.5 or 10 min after the final train of HFS and compared with control slices from the same animal in an adjacent recording chamber.B, Normalized active ERK1 immunoreactivity 2.5 min (n = 6) and 10 min (n = 4) after the washout of TEA. C, Normalized active ERK2 immunoreactivity 2.5 min (n = 6) and 10 min (n = 4) after the washout of TEA. Error bars in_B_ and C indicate SEM; * denotes statistical significance compared with control (p < 0.05 by paired Student's_t_ test).
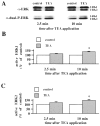
Fig. 5.
Effect of U0126 and PD 098059 on TEA-LTP.A, Ensemble averages of fEPSP slopes from slices exposed to 25 m
m
TEA for 10 min (indicated by the_horizontal_ bar) in the presence of 0.33% DMSO (open circles; _n_= 6), 20 μ
m
U0126 (open squares; n = 6), or 50 μ
m
PD 098059 (closed squares; n = 6). DMSO, U0126, or PD 098059 was present in the perfusing solution 5 min before, during, and 10 min after the washout of TEA (total time in the solution was 20 min, indicated by the horizontal bar). Both MEK inhibitors significantly blocked LTP 45 min after the washout of TEA (p < 0.001 by paired Student's_t_ test). B, Effect of U0126 on established 200 Hz-LTP. Baseline responses were recorded for 15 min before application of TEA (indicated by the horizontal bar). Twenty-five minutes after TEA application, slices were perfused with 20 μ
m
U0126 (n = 4) for 20 min. Responses were recorded for 30 min after the washout of U0126. C, Induction of TEA-LTP after the washout of U0126. Application of TEA (indicated by the horizontal bar) in the presence of 20 μ
m
U0126 (indicated by the horizontal bar) resulted in the blockade of LTP. Thirty-five minutes after the washout of U0126, TEA was applied (indicated by the horizontal bar) in the presence of 0.33% DMS0 (indicated by the_horizontal_ bar), resulting in LTP. The NMDA receptor antagonist APV (50 μ
m
) was present in the perfusing solution (indicated by the horizontal bar) in all experiments in A–C. Error bars in all panels indicate SEM.
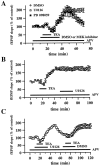
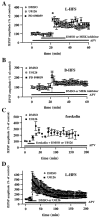
Fig. 6.
Effect of U0126 and PD 098059 on MF-LTP.A, MEK inhibitors do not block LTP induced by L-HFS (100 pulses at 100 Hz). Open circles are ensemble averages indicating the potentiation of mossy fiber fEPSP amplitudes observed after L-HFS in the presence of 0.5% DMSO (n = 5). Open _squares_are ensemble averages indicating that potentiation is observed after L-HFS in the presence of 20–50 μ
m
U0126 (n = 4). Closed squares are ensemble averages indicating that potentiation is observed after L-HFS in the presence of 20–50 μ
m
PD 098059 (n = 3). Either DMSO or the MEK inhibitor was present for 15 min before and 15 min after the HFS, as indicated by the horizontal bar_on the graph. The arrow indicates the time at which HFS was delivered. LTP was not blocked by either of the inhibitors (p > 0.3 by paired Student's_t test). B, Experiments are similar to those in A except that mossy fiber LTP was induced by B-HFS (8 pulses at 100 Hz; repeated 10 times at 5 sec intervals). Again, the MEK inhibitors (DMSO,n = 4; U0126, n = 5; PD 098059,n = 4) had no significant effect on LTP (p > 0.3 by paired Student's_t_ test). C, Slices were exposed to 50 μ
m
forskolin for 20 min (indicated by the_horizontal_ bar) in the presence of either 0.5% DMSO (open circles;n = 4) or 20 μ
m
U0126 (closed squares; n = 4). U0126 had no significant effect on forskolin-induced potentiation (p > 0.3 by paired Student's_t_ test). D, Experiments are similar to those in A except that LTP was followed for 3 hr after the L-HFS. Again, LTP was not affected by the addition of U0126 (p > 0.3 by Student's _t_test; DMSO, n = 4; U0126, n = 4). The NMDA receptor antagonist APV (50 μ
m
) was present in the perfusing solution (indicated by the horizontal bar) in all experiments in A–D. Error bars in all panels indicate SEM.
Fig. 7.
U0126 blocks associational/commissural LTP but not MF-LTP in area CA3. A, MF input. Hippocampal slices were incubated with 20 μ
m
U0126 for 30 min before recording baseline mossy fiber fEPSP amplitudes. U0126 (indicated by the_horizontal_ bar) remained in the perfusing solution for an additional 10 min before and for 30 min after the delivery of L-HFS (indicated by the arrow). The NMDA receptor antagonist APV (50 μ
m
) was present in the perfusing solution (also indicated by the horizontal bar) for the duration of the experiment. MF-LTP was not blocked by U0126. B, Associational/commissural (A/C) input. Open circles_are ensemble averages indicating the potentiation of the slope of the fEPSP observed after the delivery of HFS (indicated by the_arrow) in the presence of 0.33% DMSO (indicated by the_horizontal_ bar; n = 4). Open squares are ensemble averages indicating that potentiation is blocked after HFS in the presence of 20 μ
m
U0126 (indicated by the_horizontal_ bar; n = 4). U0126 significantly blocked LTP 45 min after the final train of HFS (p < 0.001 by paired Student's_t_ test). Error bars in all _panels_indicate SEM.
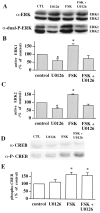
Fig. 8.
U0126 blocks forskolin-induced increases in active ERK1/ERK2 but not phospho-CREB in hippocampal area CA3.A, Representative ERK Western blots of area CA3 subregions from control slices (CTL), slices exposed to 20 μ
m
U0126, slices exposed to 50 μ
m
forskolin (FSK), and slices exposed to forskolin and U0126. B, Normalized active ERK1 immunoreactivity for each experimental condition (n = 4).C, Normalized active ERK2 immunoreactivity for each experimental condition (n = 4). D, Representative CREB Western blots of area CA3 subregions from control slices, slices exposed to 20 μ
m
U0126, slices exposed to 50 μ
m
forskolin, and slices exposed to forskolin and U0126. E, Normalized phospho-CREB immunoreactivity for each experimental condition (n = 4). Error bars in_B_, C, and E indicate SEM; * denotes statistical significance compared with control (p < 0.05 by paired Student's_t_ test with the Bonferroni correction factor).
Similar articles
- Phosphatidylinositol 3-kinase regulates the induction of long-term potentiation through extracellular signal-related kinase-independent mechanisms.
Opazo P, Watabe AM, Grant SG, O'Dell TJ. Opazo P, et al. J Neurosci. 2003 May 1;23(9):3679-88. doi: 10.1523/JNEUROSCI.23-09-03679.2003. J Neurosci. 2003. PMID: 12736339 Free PMC article. - Long-term depression in the adult hippocampus in vivo involves activation of extracellular signal-regulated kinase and phosphorylation of Elk-1.
Thiels E, Kanterewicz BI, Norman ED, Trzaskos JM, Klann E. Thiels E, et al. J Neurosci. 2002 Mar 15;22(6):2054-62. doi: 10.1523/JNEUROSCI.22-06-02054.2002. J Neurosci. 2002. PMID: 11896145 Free PMC article. - Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis.
Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Ying SW, et al. J Neurosci. 2002 Mar 1;22(5):1532-40. doi: 10.1523/JNEUROSCI.22-05-01532.2002. J Neurosci. 2002. PMID: 11880483 Free PMC article. - Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.
MacDonald JF, Jackson MF, Beazely MA. MacDonald JF, et al. Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review. - Synaptic plasticity of local connections in rat motor cortex.
Hess G. Hess G. Acta Neurobiol Exp (Wars). 2004;64(2):271-6. doi: 10.55782/ane-2004-1511. Acta Neurobiol Exp (Wars). 2004. PMID: 15366258 Review.
Cited by
- Tubb3 expression levels are sensitive to neuronal activity changes and determine microtubule growth and kinesin-mediated transport.
Radwitz J, Hausrat TJ, Heisler FF, Janiesch PC, Pechmann Y, Rübhausen M, Kneussel M. Radwitz J, et al. Cell Mol Life Sci. 2022 Oct 29;79(11):575. doi: 10.1007/s00018-022-04607-5. Cell Mol Life Sci. 2022. PMID: 36309617 Free PMC article. - Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory.
Satoh Y, Endo S, Ikeda T, Yamada K, Ito M, Kuroki M, Hiramoto T, Imamura O, Kobayashi Y, Watanabe Y, Itohara S, Takishima K. Satoh Y, et al. J Neurosci. 2007 Oct 3;27(40):10765-76. doi: 10.1523/JNEUROSCI.0117-07.2007. J Neurosci. 2007. PMID: 17913910 Free PMC article. - Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation.
Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, Mons N, Micheau J. Trifilieff P, et al. Learn Mem. 2006 May-Jun;13(3):349-58. doi: 10.1101/lm.80206. Epub 2006 May 16. Learn Mem. 2006. PMID: 16705140 Free PMC article. - Caffeine-mediated BDNF release regulates long-term synaptic plasticity through activation of IRS2 signaling.
Lao-Peregrín C, Ballesteros JJ, Fernández M, Zamora-Moratalla A, Saavedra A, Gómez Lázaro M, Pérez-Navarro E, Burks D, Martín ED. Lao-Peregrín C, et al. Addict Biol. 2017 Nov;22(6):1706-1718. doi: 10.1111/adb.12433. Epub 2016 Jul 25. Addict Biol. 2017. PMID: 27457910 Free PMC article. - Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning.
Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Schafe GE, et al. J Neurosci. 2000 Nov 1;20(21):8177-87. doi: 10.1523/JNEUROSCI.20-21-08177.2000. J Neurosci. 2000. PMID: 11050141 Free PMC article.
References
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
- Aniksztejn L, Ben-Ari Y. Novel form of long-term potentiation produced by a K+ channel blocker in the hippocampus. Nature. 1991;349:67–69. - PubMed
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. - PubMed
- Baron C, Benes C, Van Tan H, Fagard R, Roisin M-P. Potassium chloride pulse enhances mitogen-activated protein kinase activity in rat hippocampal slices. J Neurochem. 1996;66:1005–1010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS024288/NS/NINDS NIH HHS/United States
- R01 NS034007/NS/NINDS NIH HHS/United States
- R37 NS034007/NS/NINDS NIH HHS/United States
- R29 NS034007/NS/NINDS NIH HHS/United States
- NS34007/NS/NINDS NIH HHS/United States
- NS24288/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous